Yonsei Med J.
2014 May;55(3):625-634. 10.3349/ymj.2014.55.3.625.
Effect of 6% Hydroxyethyl Starch 130/0.4 as a Priming Solution on Coagulation and Inflammation Following Complex Heart Surgery
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Anam Hospital, Korea University, Seoul, Korea.
- 2Department of Anesthesiology and Pain Medicine and Anesthesia and Pain Research Institute, Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Korea. ylkwak@yuhs.ac
- KMID: 2068671
- DOI: http://doi.org/10.3349/ymj.2014.55.3.625
Abstract
- PURPOSE
Prolonged duration of cardiopulmonary bypass aggravates the degree of inflammation and coagulopathy. We investigated the influence of 6% hydroxyethyl starch (HES) 130/0.4 on coagulation and inflammation compared with albumin when used for both cardiopulmonary bypass priming and perioperative fluid therapy in patients undergoing complex valvular heart surgery.
MATERIALS AND METHODS
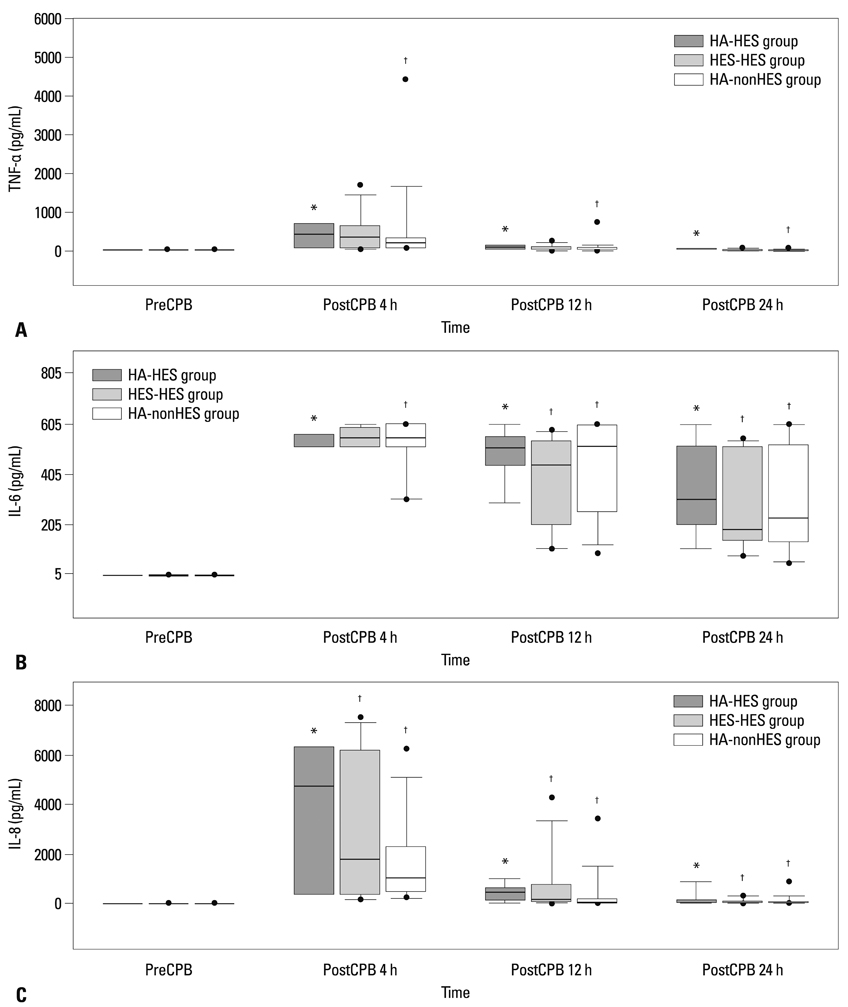
Fifty four patients were randomly allocated into albumin-HES, albumin-nonHES, and HES-HES groups. The cardiopulmonary bypass circuit was primed with 5% albumin in the albumin-HES and albumin-nonHES group, and with HES in the HES-HES group. As perioperative fluid, only plasmalyte was used in the albumin-nonHES group whereas HES was used up to 20 mL/kg in the albumin-HES and albumin-HES group. Serial assessments of coagulation profiles using the rotational thromboelastometry and inflammatory markers (tissue necrosis factor-alpha, interleukin-6, and interleukin-8) were performed.
RESULTS
Patients' characteristics and the duration of cardiopulmonary bypass (albumin-HES; 137+/-34 min, HES-HES; 136+/-47 min, albumin-nonHES; 132+/-39 min) were all similar among the groups. Postoperative coagulation profiles demonstrated sporadic increases in clot formation time and coagulation time, without any differences in the actual amount of perioperative bleeding and transfusion requirements among the groups. Also, inflammatory markers showed significant activation after cardiopulmonary bypass without any differences among the groups.
CONCLUSION
Even in the presence of prolonged duration of cardiopulmonary bypass, HES seemed to yield similar influence on the ensuing coagulopathy and inflammatory response when used for priming and perioperative fluid therapy following complex valvular heart surgery compared with conventional fluid regimen including albumin and plasmalyte.
MeSH Terms
Figure
Reference
-
1. Niemi TT, Kuitunen AH. Hydroxyethyl starch impairs in vitro coagulation. Acta Anaesthesiol Scand. 1998; 42:1104–1109.2. McCammon AT, Wright JP, Figueroa M, Nielsen VG. Hemodilution with albumin, but not Hextend, results in hypercoagulability as assessed by Thrombelastography in rabbits: role of heparin-dependent serpins and factor VIII complex. Anesth Analg. 2002; 95:844–850.
Article3. Wilkes MM, Navickis RJ, Sibbald WJ. Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: a meta-analysis of postoperative bleeding. Ann Thorac Surg. 2001; 72:527–533.
Article4. Haynes GR, Navickis RJ, Wilkes MM. Albumin administration--what is the evidence of clinical benefit? A systematic review of randomized controlled trials. Eur J Anaesthesiol. 2003; 20:771–793.
Article5. Rhee P, Wang D, Ruff P, Austin B, DeBraux S, Wolcott K, et al. Human neutrophil activation and increased adhesion by various resuscitation fluids. Crit Care Med. 2000; 28:74–78.
Article6. Vincent JL, Wilkes MM, Navickis RJ. Safety of human albumin--serious adverse events reported worldwide in 1998-2000. Br J Anaesth. 2003; 91:625–630.
Article7. Mizzi A, Tran T, Karlnoski R, Anderson A, Mangar D, Camporesi EM. Voluven, a new colloid solution. Anesthesiol Clin. 2011; 29:547–555.
Article8. Van der Linden PJ, De Hert SG, Deraedt D, Cromheecke S, De Decker K, De Paep R, et al. Hydroxyethyl starch 130/0.4 versus modified fluid gelatin for volume expansion in cardiac surgery patients: the effects on perioperative bleeding and transfusion needs. Anesth Analg. 2005; 101:629–634.
Article9. Kasper SM, Meinert P, Kampe S, Görg C, Geisen C, Mehlhorn U, et al. Large-dose hydroxyethyl starch 130/0.4 does not increase blood loss and transfusion requirements in coronary artery bypass surgery compared with hydroxyethyl starch 200/0.5 at recommended doses. Anesthesiology. 2003; 99:42–47.
Article10. Vlaar AP, Hofstra JJ, Determann RM, Veelo DP, Paulus F, Levi M, et al. Transfusion-related acute lung injury in cardiac surgery patients is characterized by pulmonary inflammation and coagulopathy: a prospective nested case-control study. Crit Care Med. 2012; 40:2813–2820.
Article11. Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011; 7:201–208.
Article12. Tanaka KA, Bolliger D, Vadlamudi R, Nimmo A. Rotational thromboelastometry (ROTEM)-based coagulation management in cardiac surgery and major trauma. J Cardiothorac Vasc Anesth. 2012; 26:1083–1093.
Article13. Schramko AA, Suojaranta-Ylinen RT, Kuitunen AH, Kukkonen SI, Niemi TT. Rapidly degradable hydroxyethyl starch solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. Anesth Analg. 2009; 108:30–36.
Article14. Westphal M, James MF, Kozek-Langenecker S, Stocker R, Guidet B, Van Aken H. Hydroxyethyl starches: different products--different effects. Anesthesiology. 2009; 111:187–202.15. Kuitunen AH, Hynynen MJ, Vahtera E, Salmenperä MT. Hydroxyethyl starch as a priming solution for cardiopulmonary bypass impairs hemostasis after cardiac surgery. Anesth Analg. 2004; 98:291–297.
Article16. Rackow EC, Falk JL, Fein IA, Siegel JS, Packman MI, Haupt MT, et al. Fluid resuscitation in circulatory shock: a comparison of the cardiorespiratory effects of albumin, hetastarch, and saline solutions in patients with hypovolemic and septic shock. Crit Care Med. 1983; 11:839–850.17. Niemi TT, Kuitunen AH. Artificial colloids impair haemostasis. An in vitro study using thromboelastometry coagulation analysis. Acta Anaesthesiol Scand. 2005; 49:373–378.
Article18. Nakahira A, Sasaki Y, Hirai H, Fukui T, Matsuo M, Takahashi Y, et al. Closed cardiopulmonary bypass circuits suppress thrombin generation during coronary artery bypass grafting. Interact Cardiovasc Thorac Surg. 2010; 10:555–560.
Article19. Karkouti K, McCluskey SA, Syed S, Pazaratz C, Poonawala H, Crowther MA. The influence of perioperative coagulation status on postoperative blood loss in complex cardiac surgery: a prospective observational study. Anesth Analg. 2010; 110:1533–1540.
Article20. Paparella D, Brister SJ, Buchanan MR. Coagulation disorders of cardiopulmonary bypass: a review. Intensive Care Med. 2004; 30:1873–1881.
Article21. Apostolakis EE, Koletsis EN, Baikoussis NG, Siminelakis SN, Papadopoulos GS. Strategies to prevent intraoperative lung injury during cardiopulmonary bypass. J Cardiothorac Surg. 2010; 5:1.
Article22. Sander M, Spies CD, Martiny V, Rosenthal C, Wernecke KD, von Heymann C. Mortality associated with administration of high-dose tranexamic acid and aprotinin in primary open-heart procedures: a retrospective analysis. Crit Care. 2010; 14:R148.
Article23. Choi YS, Shim JK, Hong SW, Kim JC, Kwak YL. Comparing the effects of 5% albumin and 6% hydroxyethyl starch 130/0.4 on coagulation and inflammatory response when used as priming solutions for cardiopulmonary bypass. Minerva Anestesiol. 2010; 76:584–591.24. Adrian K, Mellgren K, Skogby M, Friberg LG, Mellgren G, Wadenvik H. The effect of albumin priming solution on platelet activation during experimental long-term perfusion. Perfusion. 1998; 13:187–191.
Article25. Kamada T, McMillan DE, Sternlieb JJ, Björk VO, Otsuji S. Albumin prevents erythrocyte crenation in patients undergoing extracorporeal circulation. Scand J Thorac Cardiovasc Surg. 1988; 22:155–158.
Article26. Ebinger T, Ruland A, Lakner M, Schwaiger M. Validity, regulatory registration and approval of ROTEM thromboelastometry. Blood Coagul Fibrinolysis. 2010; 21:106–107.
Article27. Leemann H, Lustenberger T, Talving P, Kobayashi L, Bukur M, Brenni M, et al. The role of rotation thromboelastometry in early prediction of massive transfusion. J Trauma. 2010; 69:1403–1408.28. Roullet S, Pillot J, Freyburger G, Biais M, Quinart A, Rault A, et al. Rotation thromboelastometry detects thrombocytopenia and hypofibrinogenaemia during orthotopic liver transplantation. Br J Anaesth. 2010; 104:422–428.
Article29. Theusinger OM, Wanner GA, Emmert MY, Billeter A, Eismon J, Seifert B, et al. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM) is associated with higher mortality in patients with severe trauma. Anesth Analg. 2011; 113:1003–1012.
Article30. Wan S, Izzat MB, Lee TW, Wan IY, Tang NL, Yim AP. Avoiding cardiopulmonary bypass in multivessel CABG reduces cytokine response and myocardial injury. Ann Thorac Surg. 1999; 68:52–56.
Article31. Zarychanski R, Abou-Setta AM, Turgeon AF, Houston BL, McIntyre L, Marshall JC, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013; 309:678–688.
Article32. Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, et al. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012; 367:1901–1911.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to "Effect of 6% Hydroxyethyl Starch 130/0.4 as a Priming Solution on Coagulation and Inflammation Following Complex Heart Surgery" by Cho JE, et al. (Yonsei Med J 2014;55:625-34.)
- Effect of hydroxyethyl starch on blood glucose levels
- A Comparison between 20% Albumin and 10% Pentastarch in Priming fluid for Cardiopulmonary bypass
- Effects of Intravascular Volume Therapy with a Hydroxyethyl Starch (HES 130/0.4) on Blood Coagulation in Children Undergoing Cardiac Surgery
- In Vitro Coagulation Study of Hemodiluted Blood with Hydroxyethyl Starch by Thromboelastography