J Korean Med Assoc.
2006 May;49(5):410-415. 10.5124/jkma.2006.49.5.410.
Transfusion-Transmitted Diseases: Current State and Recent Countermeasures
- Affiliations
-
- 1Blood Safety Division of Blood Service Headquarters, Korean Red Cross, Korea. seo2023@redcross.or.kr
- KMID: 2064948
- DOI: http://doi.org/10.5124/jkma.2006.49.5.410
Abstract
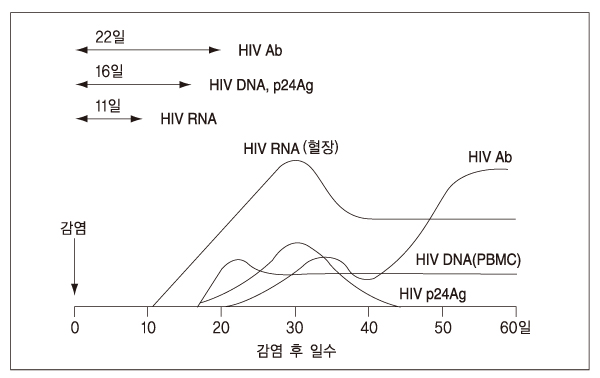
- Infectious agents, including viruses, bacteria and parasites, can be transmitted via human blood and blood products. Of greatest importance are viruses such as human immunodeficiency virus types 1 and 2 (HIV-1/2), hepatitis B virus (HBV), and hepatitis C virus (HCV), followed by other viruses such as cytomegalovirus (CMV) and human parvovirus B19. Viruses such as hepatitis G virus and TT virus can also be transmitted via blood products, but their pathogenicity is still unclear. Bacteria, including Treponema pallidum and Yersinia enterocolitica and parasites such as Plasmodium species can also be transmitted from donors to recipients. Furthermore, the threat of newly emerging pathogens that can affect the blood safety, such as the variant Creutzfeld-Jakob Disease, is always present. The measures to reduce the risks of transfusiontransmitted infection within the last 20 years, such as donor selection and testing donated blood for various infectious agents, have had a remarkable impact on the safety of blood supply. Nevertheless, the public expectation of absolute blood safety continues to exert pressure to eliminate the remaining risks. The recent introduction of molecular biology techniques combined with viral inactivation methods is directed to get this goal.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Evaluation of HBs Ag, HCV and HIV Ag-Ab Assays using Bio-Rad Elite Microplate Analyzer
Sang-Hyun Hwang, Heung-Bum Oh, Hyon-Suk Kim, Eun Yup Lee
Korean J Lab Med. 2006;26(6):436-441. doi: 10.3343/kjlm.2006.26.6.436.
Reference
-
2. Louisirirotchanakul S, Kanoksinsombat C, Theamboonlert A, Puthavatana P, Wasi C, Poovorawan Y. Mutation of the "a" determinant of HBsAg with discordant HBsAg diagnostic kits. Viral Immunology. 2004. 17:440–444.
Article3. Klein HG, Anstee DJ. Mollison's blood transfusion in clinical medicine. 2005. 11th ed. Oxford: Blackwell Publishing;701–773.4. Donegan E, Stuart M, Niland JC. Transfusion Safety Study Group. Infection with human immunodeficiency virus type I (HIV-1) among recipients of antibody-positive blood donations. Ann Intern Med. 1990. 113:733.
Article7. Llewelyn CA, Hewitt PE, Knight RSG, Amar K, Cousens S, Mackenzie J, et al. Possible transmission of variant Creutzfeldt-Jakob disease by blood transfusion. Lancet. 2004. 363:417–421.
Article8. Ludlam CA, Turner ML. Managing the risk of transmission of variant Creutzfeldt-Jakob disease by blood products. British Journal of Haematology. 2005. 132:13–24.
Article9. Guidance for Industry, Revised preventive measures to reduce the possible risk of transmission of Creutzfeldt-Jakob Disease (CJD) and Variant Creutzfeldt-Jakob Disease (vCJD) by Blood and Blood Products. http://www.fda.gov/cber/gdlns/cjdvcjd.pdf.