Obstet Gynecol Sci.
2015 Sep;58(5):391-396. 10.5468/ogs.2015.58.5.391.
Expression of angiogenic factors in cryopreserved mouse ovaries after heterotopic autotransplantation
- Affiliations
-
- 1Department of Obstetrics and Gynecology, School of Medicine, Gyeongsang National University, Jinju, Korea. wypaik@gnu.ac.kr
- 2Institute of Health Sciences, Gyeongsang National University, Jinju, Korea.
- KMID: 2058463
- DOI: http://doi.org/10.5468/ogs.2015.58.5.391
Abstract
OBJECTIVE
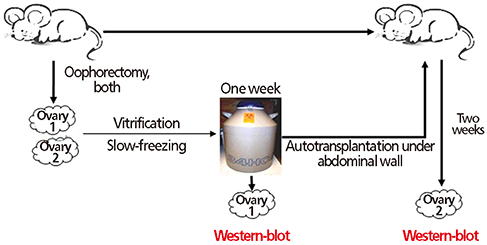
Revascularization is critical for successful ovarian tissue transplantation. Vascular endothelial growth factor (VEGF) and angiopoietin-2 (angpt-2) are the principal mediators of neovascularization. This study was designed to assess VEGF and angpt-2 levels in cryopreserved ovarian tissue after heterotopic autotransplantation.
METHODS
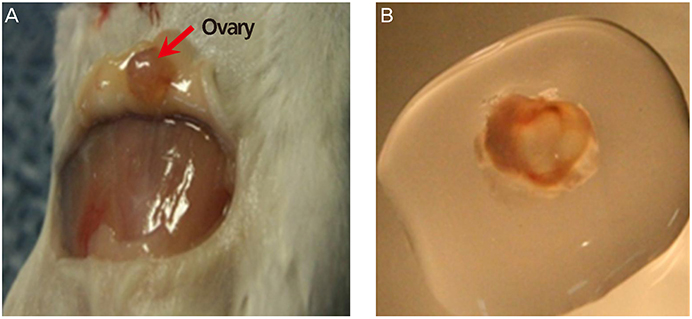
Ovarian tissues harvested from ICR mice at 5 to 6 weeks of age were stratified as follows: no cryopreservation (controls, group I); vitrification in VFS-40 (vitrification, group II); and gradual freezing in dimethyl sulfoxide (slow-freezing, group III). Frozen specimens were thawed at room temperature, assaying VEGF and angpt-2 levels 1 week after cryopreservation and 2 weeks after autotransplantation.
RESULTS
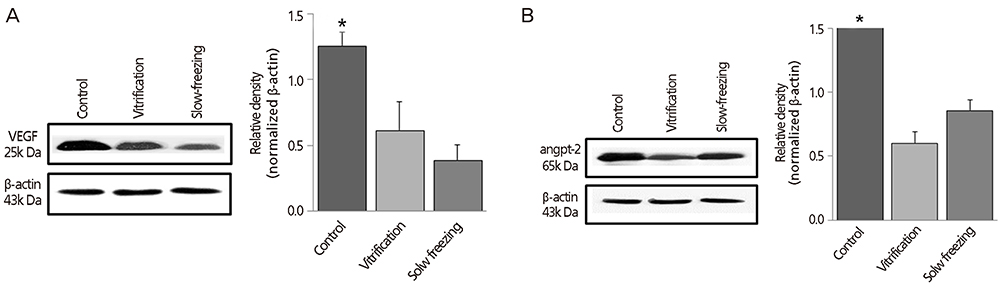
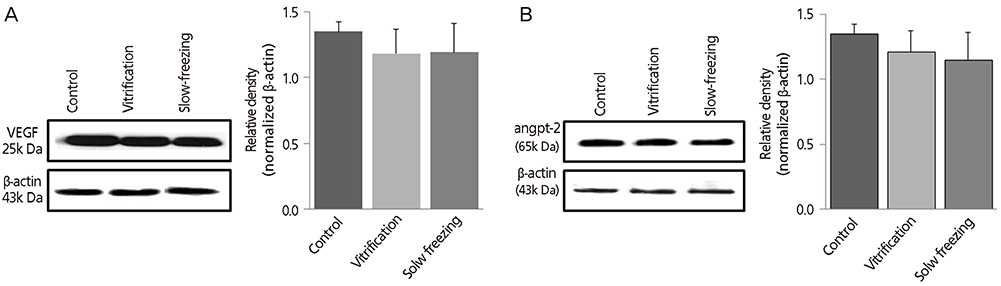
VEGF and angpt-2 protein levels were significantly lower in cryopreserved ovaries of groups II and III than in controls (group I, P<0.05), whereas groups II and III did not differ significantly in this regard. After autotransplantation of cryopreserved ovarian tissue, VEGF and angpt-2 protein levels did not differ significantly by technique but tended to be lower than corresponding levels in controls.
CONCLUSION
Expression of angiogenic factors in ovarian tissue is thought to vary by method of cryopreservation. Our findings indicate that levels of angiogenic factors expressed in cryopreserved ovarian tissue after autotransplantation do not differ appreciably from control levels, regardless of cryopreservation technique.
Keyword
MeSH Terms
-
Angiogenesis Inducing Agents*
Angiopoietin-2
Animals
Autografts*
Cryopreservation
Dimethyl Sulfoxide
Female
Freezing
Mice*
Mice, Inbred ICR
Ovary*
Tissue Transplantation
Transplantation
Transplants
Vascular Endothelial Growth Factor A
Vitrification
Angiogenesis Inducing Agents
Angiopoietin-2
Dimethyl Sulfoxide
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Blatt J. Pregnancy outcome in long-term survivors of childhood cancer. Med Pediatr Oncol. 1999; 33:29–33.2. Aubard Y, Poirot C, Piver P, Galinat S, Teissier MP. Are there indications for ovarian tissue cryopreservation? Fertil Steril. 2001; 76:414–415.3. Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. JAMA. 2001; 286:1490–1493.4. Oktay K. Successful human ovarian autotransplantation to the upper arm. Cancer. 2005; 103:1982–1983.5. Kim SS, Hwang IT, Lee HC. Heterotopic autotransplantation of cryobanked human ovarian tissue as a strategy to restore ovarian function. Fertil Steril. 2004; 82:930–932.6. Bedaiwy MA, Jeremias E, Gurunluoglu R, Hussein MR, Siemianow M, Biscotti C, et al. Restoration of ovarian function after autotransplantation of intact frozen-thawed sheep ovaries with microvascular anastomosis. Fertil Steril. 2003; 79:594–602.7. Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004; 363:837–840.8. Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000; 407:242–248.9. Hazzard TM, Molskness TA, Chaffin CL, Stouffer RL. Vascular endothelial growth factor (VEGF) and angiopoietin regulation by gonadotrophin and steroids in macaque granulosa cells during the peri-ovulatory interval. Mol Hum Reprod. 1999; 5:1115–1121.10. Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999; 13:1055–1066.11. Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996; 87:1171–1180.12. Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000; 6:460–463.13. Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999; 18:5356–5362.14. Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998; 83:233–240.15. Nishigaki A, Okada H, Tsuzuki T, Cho H, Yasuda K, Kanzaki H. Angiopoietin 1 and angiopoietin 2 in follicular fluid of women undergoing a long protocol. Fertil Steril. 2011; 96:1378–1383.16. Choi WJ, Lee JH, Park MH, Choi IY, Park JK, Shin JK, et al. Influence of the vitrification solution on the angiogenic factors in vitrificated mouse ovarian tissue. Obstet Gynecol Sci. 2013; 56:382–388.17. Maltaris T, Beckmann MW, Dittrich R. Fertility preservation for young female cancer patients. In Vivo. 2009; 23:123–130.18. Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012; 18:59–67.19. Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Long-term ovarian function in sheep after ovariectomy and transplantation of autografts stored at -196 C. Endocrinology. 1999; 140:462–471.20. Abir R, Orvieto R, Raanani H, Feldberg D, Nitke S, Fisch B. Parameters affecting successful transplantation of frozenthawed human fetal ovaries into immunodeficient mice. Fertil Steril. 2003; 80:421–428.21. Israely T, Dafni H, Nevo N, Tsafriri A, Neeman M. Angiogenesis in ectopic ovarian xenotransplantation: multiparameter characterization of the neovasculature by dynamic contrastenhanced MRI. Magn Reson Med. 2004; 52:741–750.22. Kim SS, Yang HW, Kang HG, Lee HH, Lee HC, Ko DS, et al. Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril. 2004; 82:679–685.23. Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Microbiol Immunol. 1999; 237:1–30.24. Friedman O, Orvieto R, Fisch B, Felz C, Freud E, Ben-Haroush A, et al. Possible improvements in human ovarian grafting by various host and graft treatments. Hum Reprod. 2012; 27:474–482.25. Isachenko V, Isachenko E, Reinsberg J, Montag M, van der Ven K, Dorn C, et al. Cryopreservation of human ovarian tissue: comparison of rapid and conventional freezing. Cryobiology. 2007; 55:261–268.26. Santos RR, Tharasanit T, Van Haeften T, Figueiredo JR, Silva JR, Van den Hurk R. Vitrification of goat preantral follicles enclosed in ovarian tissue by using conventional and solid-surface vitrification methods. Cell Tissue Res. 2007; 327:167–176.27. Dissen GA, Lara HE, Fahrenbach WH, Costa ME, Ojeda SR. Immature rat ovaries become revascularized rapidly after autotransplantation and show a gonadotropin-dependent increase in angiogenic factor gene expression. Endocrinology. 1994; 134:1146–1154.28. Bedaiwy MA, Falcone T. Harvesting and autotransplantation of vascularized ovarian grafts: approaches and techniques. Reprod Biomed Online. 2007; 14:360–371.29. Jeremias E, Bedaiwy MA, Gurunluoglu R, Biscotti CV, Siemionow M, Falcone T. Heterotopic autotransplantation of the ovary with microvascular anastomosis: a novel surgical technique. Fertil Steril. 2002; 77:1278–1282.30. Grazul-Bilska AT, Banerjee J, Yazici I, Borowczyk E, Bilski JJ, Sharma RK, et al. Morphology and function of cryopreserved whole ovine ovaries after heterotopic autotransplantation. Reprod Biol Endocrinol. 2008; 6:16.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of SDF-1alpha and leptin, and their effect on expression of angiogenic factors in mouse ovaries
- Review of Heterotopic Thyroid Autotransplantation
- Restoration of Reproductive Potential after Autotransplantation of Frozen Ovaries in Mice Pretreated with Cyclophosphamide
- Effect of Cryopreservation on the Heat Shock Protein 90 Expression in Mouse Ovarian Tissue
- Influence of the vitrification solution on the angiogenic factors in vitrificated mouse ovarian tissue