J Gynecol Oncol.
2009 Mar;20(1):48-54. 10.3802/jgo.2009.20.1.48.
Synergistic growth inhibition by combination of adenovirus mediated p53 transfer and cisplatin in ovarian cancer cell lines
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea Cancer Center Hospital, Seoul, Korea.
- 2Department of Laboratory of Molecular Biology, Korea Cancer Center Hospital, Seoul, Korea.
- 3Department of Internal Medicine, National Cancer Center, Goyang, Korea.
- 4Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea. ksboo308@plaza.snu.ac.kr
- KMID: 2055649
- DOI: http://doi.org/10.3802/jgo.2009.20.1.48
Abstract
OBJECTIVE
This study was to investigate the synergistic growth inhibitory effect by combination of adenovirus mediated p53 gene transfer and cisplatin in ovarian cancer cell lines with different p53 gene mutation patterns.
METHODS
Three ovarian cancer cell lines, p53 deleted SKOV3, p53 mutated OVCAR-3, and PA-1 with wild-type p53 were transduced with human adenovirus vectors carrying p53 gene (Ad-p53) and treated with a sublethal concentration of cisplatin before and after Ad-p53. The cell number was counted daily for 5 days after Ad-p53 transduction. Western blotting was used to identify p53 and p21 protein expressions, and flow cytometric analysis was performed to investigate any change of DNA ploidy after Ad-p53 transfer.
RESULTS
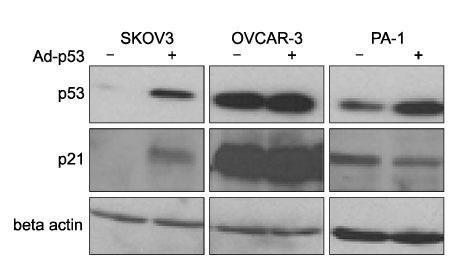
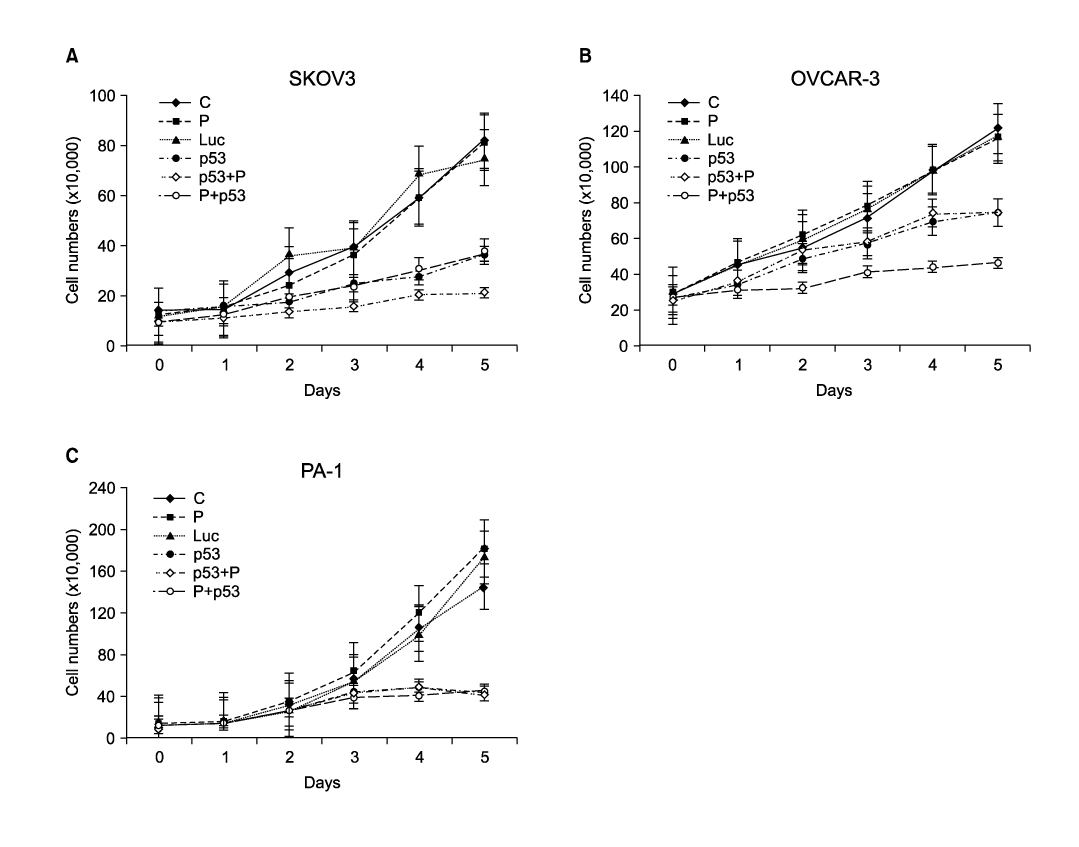
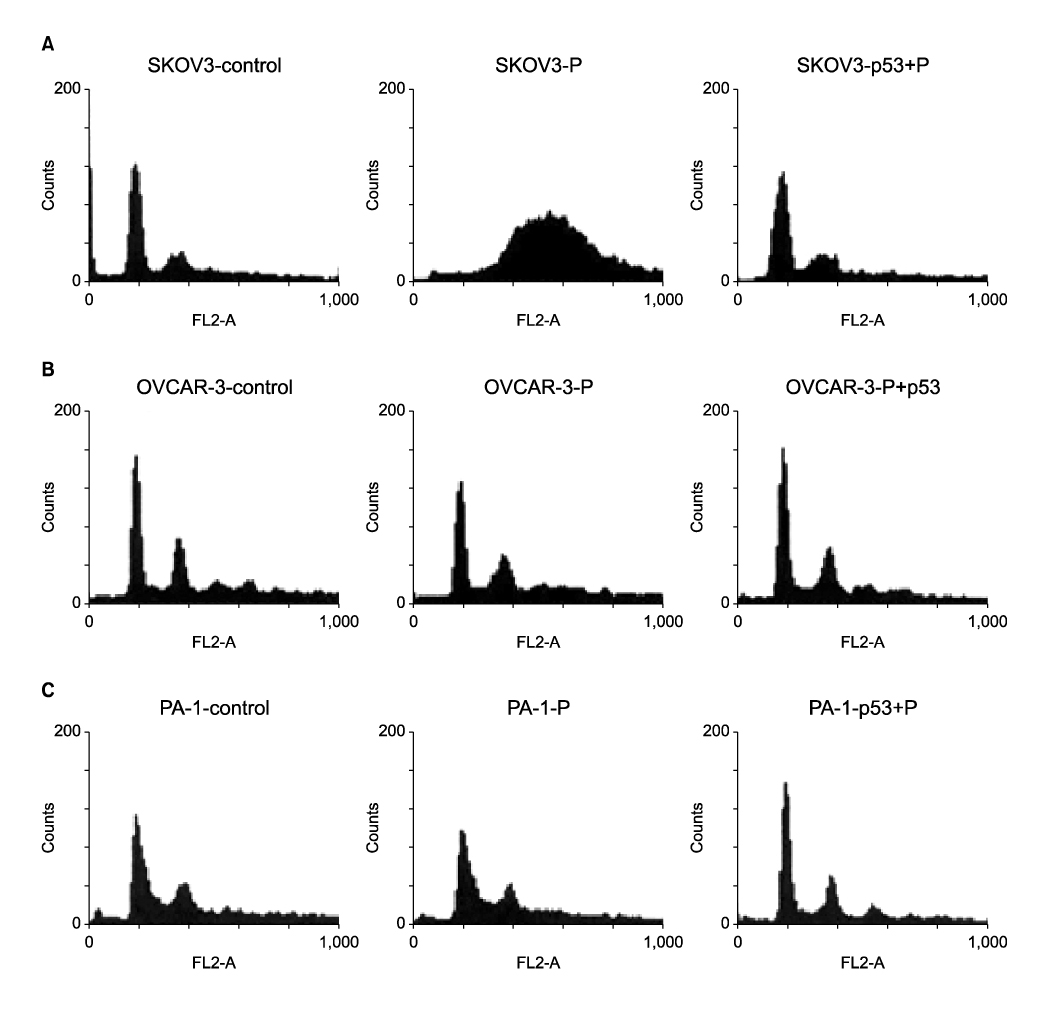
Ad-p53 transduced cells successfully expressed p53 and p21 proteins after 48 hours of Ad-p53 transduction. Synergistic growth inhibition by combination of Ad-p53 and cisplatin was detected only in SKOV3 and OVCAR-3 cells, but not in PA-1 cells. In p53 deleted SKOV3 cells, cisplatin treatment after Ad-p53 showed higher growth inhibition than the treatment before Ad-p53 transduction, and reverse relationship was observed in p53 mutated OVCAR-3 cells. In SKOV3 cells, the fraction of cells at G2/M phase increased after cisplatin treatment, however, it decreased dramatically with Ad-p53 transduction.
CONCLUSION
The synergistic growth inhibition by combination of Ad-p53 and cisplatin may depend on the p53 status and the temporal sequence of cisplatin treatment, suggesting judicious selective application of this strategy in clinical trials.
Keyword
MeSH Terms
Figure
Reference
-
1. Ozols RF, Schwartz PE, Eifel PJ. DeVita VT, Hellman S, Rosenberg SA, editors. Ovarian cancer, fallopian tube carcinoma, and peritoneal carcinoma. Cancer: Principle and practice of oncology. 1997. 5th ed. Philadelphia: Lippincott-Raven Publishers;1502–1539.2. Pecorelli S, Odicino F, Maisonneuve P, Creasman W, Shepard J, Sideri M, et al. Carcinoma of the ovary. J Epidemiol Biostat. 1998. 3:75–102.3. Hamilton TC, Johnson SW, Godwin AK. Ozols RF, editor. Molecular biology of gynecologic malignancies. Gynecologic oncology. 1998. 1st ed. Boston: Kluwer Academic Publisher;103–114.4. Kohler MF, Marks JR, Wiseman RW, Jacobs IJ, Davidoff AM, Clarke-Pearson DL, et al. Spectrum of mutation and frequency of allelic deletion of the p53 gene in ovarian cancer. J Natl Cancer Inst. 1993. 85:1513–1519.5. Kupryjanczyk J, Thor AD, Beauchamp R, Merritt V, Edgerton SM, Bell DA, et al. p53 gene mutations and protein accumulation in human ovarian cancer. Proc Natl Acad Sci U S A. 1993. 90:4961–4965.6. Berchuck A, Kohler MF, Bast RC Jr. Molecular genetic features of ovarian cancer. Prog Clin Biol Res. 1996. 394:269–284.7. Chen PL, Chen YM, Bookstein R, Lee WH. Genetic mechanisms of tumor suppression by the human p53 gene. Science. 1990. 250:1576–1580.8. Williams GT, Smith CA. Molecular regulation of apoptosis: Genetic controls on cell death. Cell. 1993. 74:777–779.9. Shaw P, Bovey R, Tardy S, Sahli R, Sordat B, Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci U S A. 1992. 89:4495–4499.10. Eastham JA, Hall SJ, Sehgal I, Wang J, Timme TL, Yang G, et al. In vivo gene therapy with p53 or p21 adenovirus for prostate cancer. Cancer Res. 1995. 55:5151–5155.11. Roth JA, Nguyen D, Lawrence DD, Kemp BL, Carrasco CH, Ferson DZ, et al. Retrovirus-mediated wild-type p53 gene transfer to tumors of patients with lung cancer. Nat Med. 1996. 2:985–991.12. Mujoo K, Maneval DC, Anderson SC, Gutterman JU. Adenoviral-mediated p53 tumor suppressor gene therapy of human ovarian carcinoma. Oncogene. 1996. 12:1617–1623.13. Polyak K, Waldman T, He TC, Kinzler KW, Vogelstein B. Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 1996. 10:1945–1952.14. von Gruenigen VE, Santoso JT, Coleman RL, Muller CY, Miller DS, Mathis JM. In vivo studies of adenovirus-based p53 gene therapy for ovarian cancer. Gynecol Oncol. 1998. 69:197–204.15. Yang B, Eshleman JR, Berger NA, Markowitz SD. Wild-type p53 protein potentiates cytotoxicity of therapeutic agents in human colon cancer cells. Clin Cancer Res. 1996. 2:1649–1657.16. Fujiwara T, Grimm EA, Mukhopadhyay T, Zhang WW, Owen-Schaub LB, Roth JA. Induction of chemosensitivity in human lung cancer cells in vivo by adenovirus-mediated transfer of the wild-type p53 gene. Cancer Res. 1994. 54:2287–2291.17. Kanamori Y, Kigawa J, Minagawa Y, Irie T, Oishi T, Shimada M, et al. A newly developed adenovirus-mediated transfer of a wild-type p53 gene increases sensitivity to cis-diamminedichloroplatinum (II) in p53-deleted ovarian cancer cells. Eur J Cancer. 1998. 34:1802–1806.18. McGuire WP, Hoskins WJ, Brady MF, Homesley HD, Creasman WT, Berman ML, et al. Assessment of dose-intensive therapy in suboptimally debulked ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 1995. 13:1589–1599.19. Gruppo Interegionale Cooperativo Oncologico Ginecologia. Randomised comparison of cisplatin with cyclophosphamide/cisplatin and with cyclophosphamide/doxorubicin/cisplatin in advanced ovarian cancer. Lancet. 1987. 2:353–359.20. Ogawa N, Fujiwara T, Kagawa S, Nishizaki M, Morimoto Y, Tanida T, et al. Novel combination therapy for human colon cancer with adenovirus-mediated wild-type p53 gene transfer and DNA-damaging chemotherapeutic agent. Int J Cancer. 1997. 73:367–370.21. Nguyen DM, Spitz FR, Yen N, Cristiano RJ, Roth JA. Gene therapy for lung cancer: enhancement of tumor suppression by a combination of sequential systemic cisplatin and adenovirus-mediated p53 gene transfer. J Thorac Cardiovasc Surg. 1996. 112:1372–1376.22. Hamada M, Fujiwara T, Hizuta A, Gochi A, Naomoto Y, Takakura N, et al. The p53 gene is a potent determinant of chemosensitivity and radiosensitivity in gastric and colorectal cancers. J Cancer Res Clin Oncol. 1996. 122:360–365.23. Cross SM, Sanchez CA, Morgan CA, Schimke MK, Ramel S, Idzerda RL, et al. A p53-dependent mouse spindle checkpoint. Science. 1995. 267:1353–1356.24. Kastan MB, Canman CE, Leonard CJ. P53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev. 1995. 14:3–15.25. Waldman T, Lengauer C, Kinzler KW, Vogelstein B. Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature. 1996. 381:713–716.26. Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998. 282:1497–1501.27. Duesberg P, Rausch C, Rasnick D, Hehlmann R. Genetic instability of cancer cells is proportional to their degree of aneuploidy. Proc Natl Acad Sci U S A. 1998. 95:13692–13697.28. Yaginuma Y, Westphal H. Abnormal structure and expression of the p53 gene in human ovarian carcinoma cell lines. Cancer Res. 1992. 52:4196–4199.29. Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977. 36:59–74.30. Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 1989. 2nd ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.31. Kanegae Y, Makimura M, Saito I. A simple and efficient method for purification of infectious recombinant adenovirus. Jpn J Med Sci Biol. 1994. 47:157–166.32. Tang DC, Johnston SA, Carbone DP. Butyrate-inducible and tumor-restricted gene expression by adenovirus vectors. Cancer Gene Ther. 1994. 1:15–20.33. Skehan P. Julio EC, editor. Cytotoxicity and cell growth assays. Cell biology. 1998. 2nd ed. San Diego: Academic Press;313–318.34. Juan G, Darzynkiewicz Z. Julio EC, editor. Cell cycle analysis by flow and laser scanning cytometry. Cell biology. 1998. 2nd ed. San Diego: Academic Press;261–274.35. Zhang WW, Fang X, Mazur W, French BA, Georges RN, Roth JA. High-efficiency gene transfer and high-level expression of wild-type p53 in human lung cancer cells mediated by recombinant adenovirus. Cancer Gene Ther. 1994. 1:5–13.36. Roemer K, Friedmann T. Mechanisms of action of the p53 tumor suppressor and prospects for cancer gene therapy by reconstitution of p53 function. Ann N Y Acad Sci. 1994. 716:265–280.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Restoration of Wild-Type p53 by Adenovirus-Mediated Gene Transfer May Enhance the Therapeutic Efficacy of Chemotherapy in Human Ovarian Cancer Cells
- Enhanced Growth inhibition by Combined Gene Transfer of p53 and p16INK4a in Adenoviral Vectors to Lung Cancer Cell Lines
- Synergistic Effect of Theophylline and Cisplatin on Inhibition of Cell Growth and Induction of Apoptosis in Human Epithelial Ovarian Cancer Cells
- p53 gene transfer does not enhance E2F-1-mediated apoptosis in human colon cancer cells
- Expression of p73 in Null-p53 SKOV3 Ovarian Cancer Cell Line