J Bacteriol Virol.
2006 Sep;36(3):151-157. 10.4167/jbv.2006.36.3.151.
Adhesion of Weissella cibaria to the Epithelial Cells and Factors Affecting its Adhesion
- Affiliations
-
- 1Department of Microbiology and Immunology, School of Medicine, Chonnam National University, Gwangju, Korea. joh@chonnam.ac.kr
- KMID: 2055022
- DOI: http://doi.org/10.4167/jbv.2006.36.3.151
Abstract
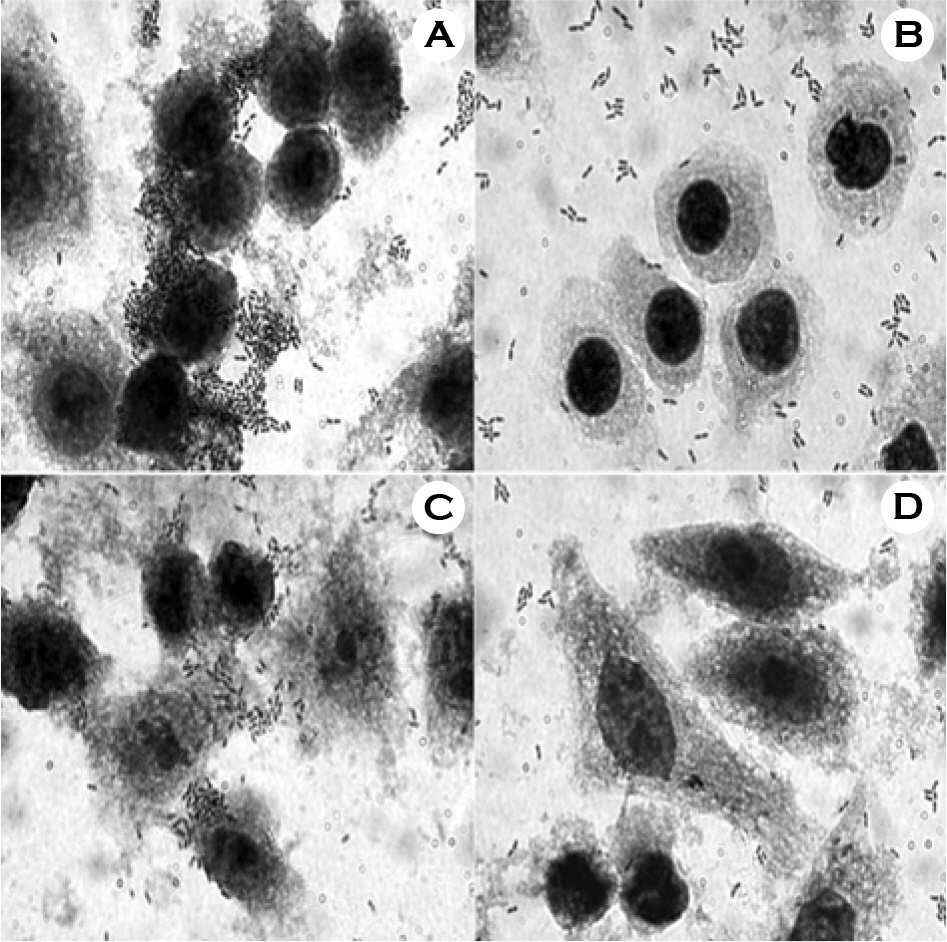
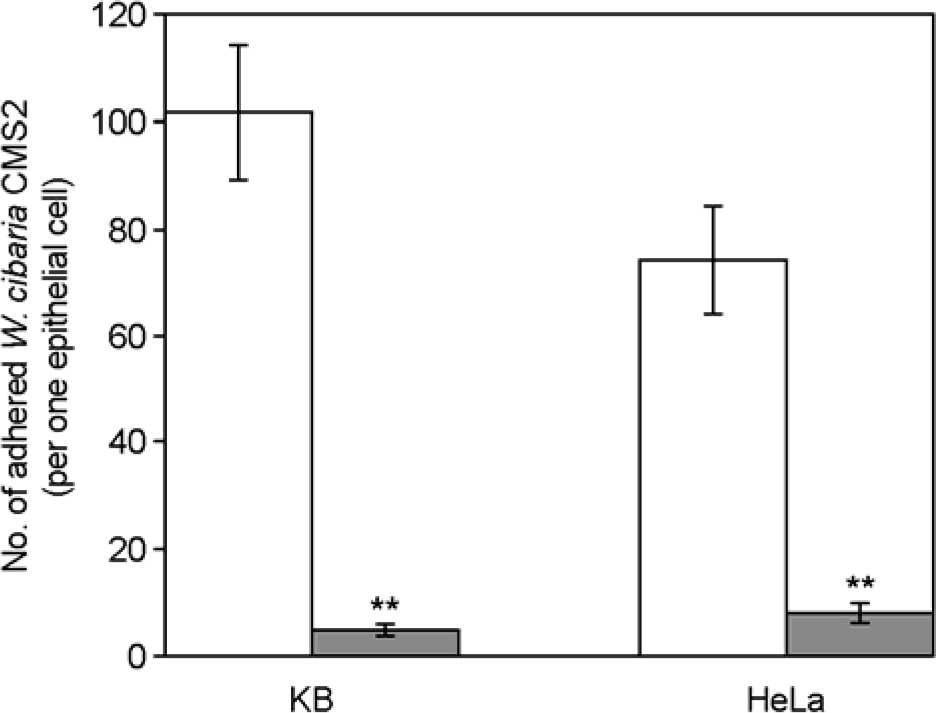
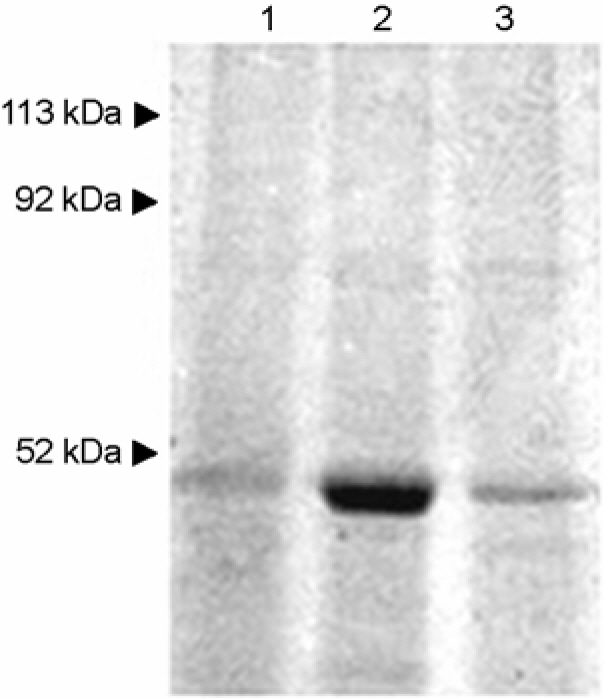
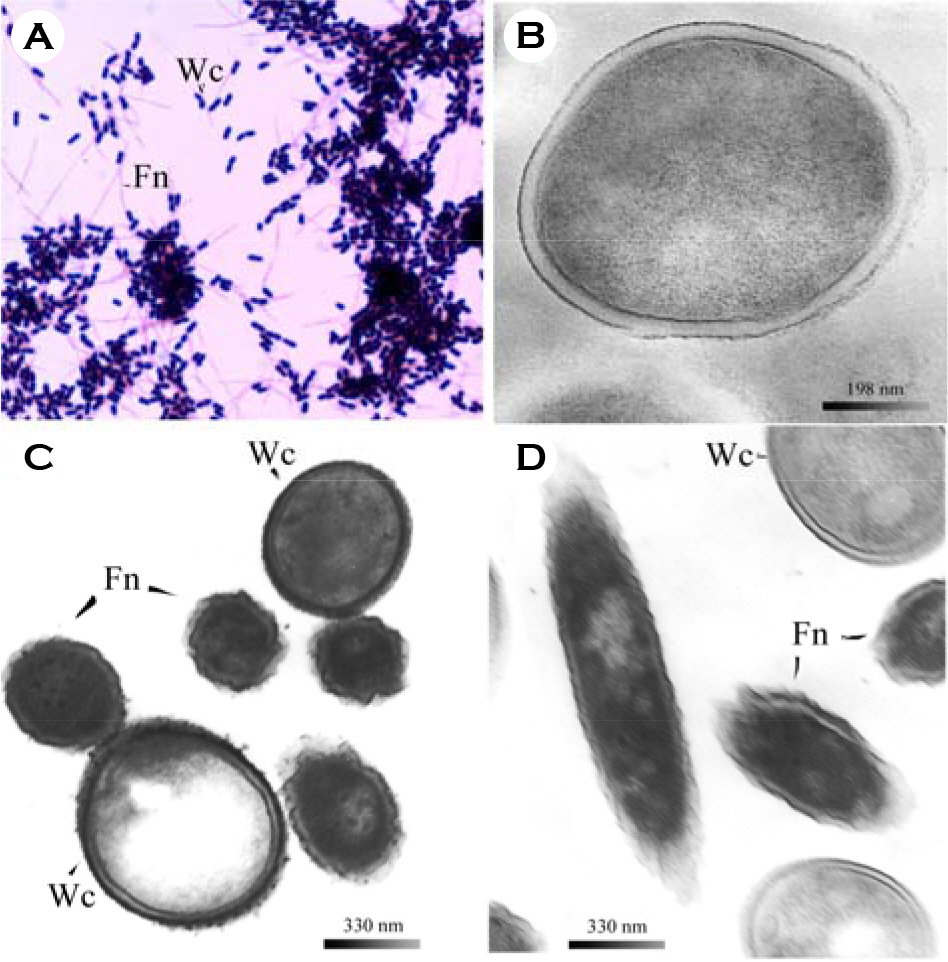
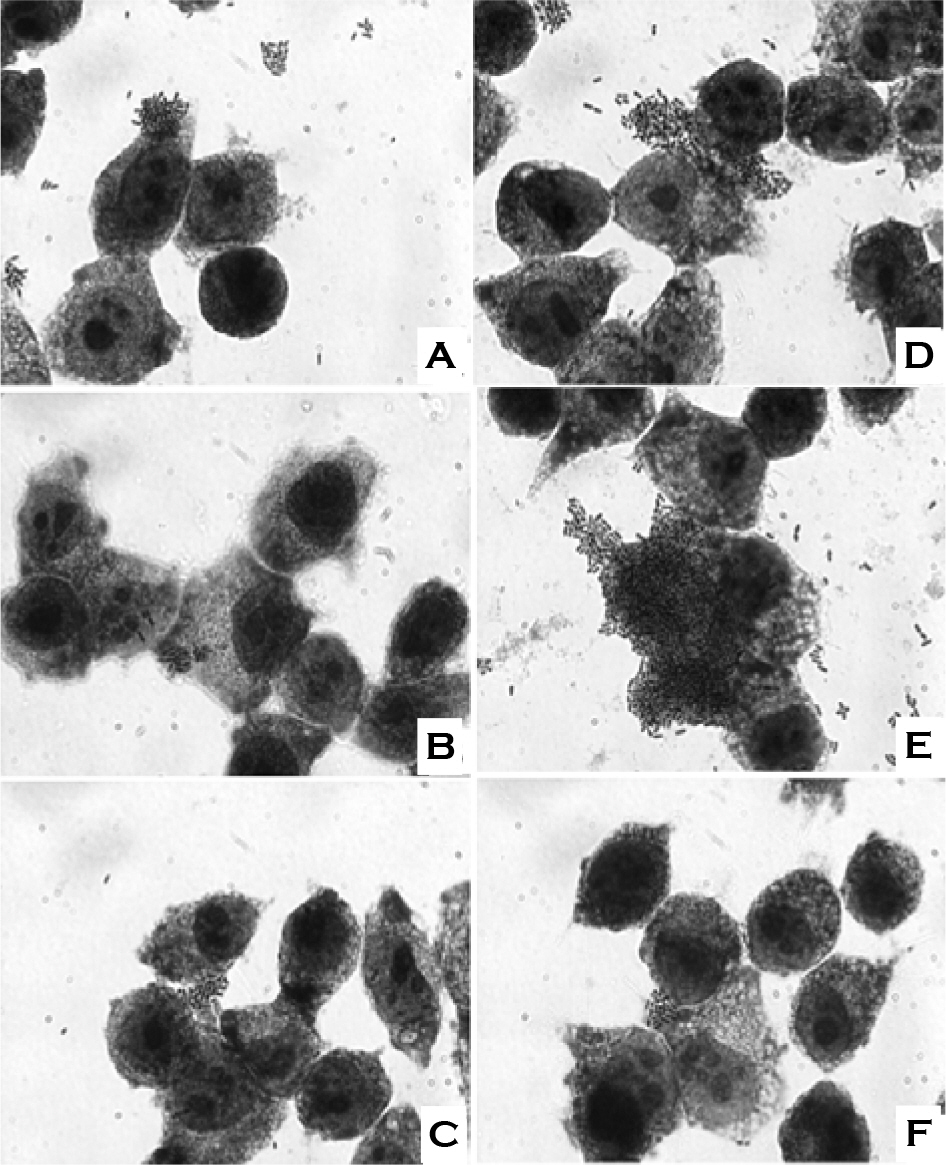
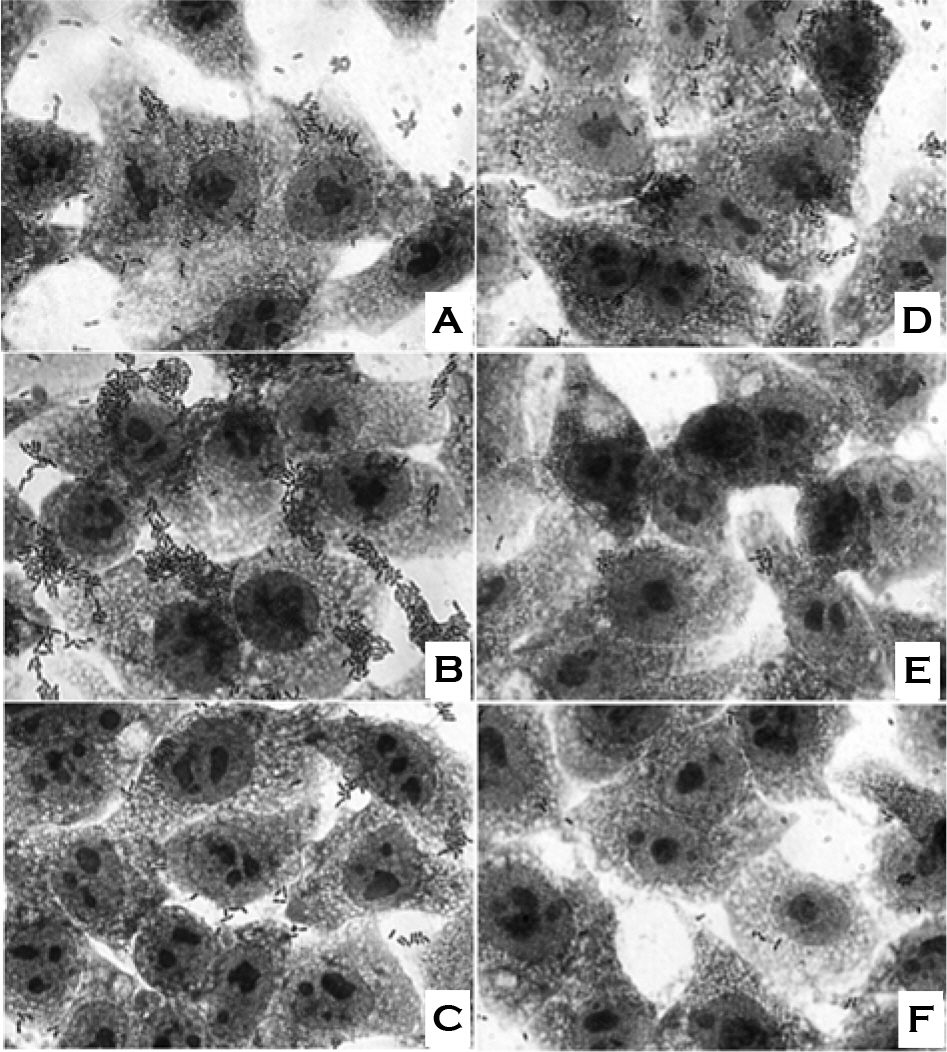
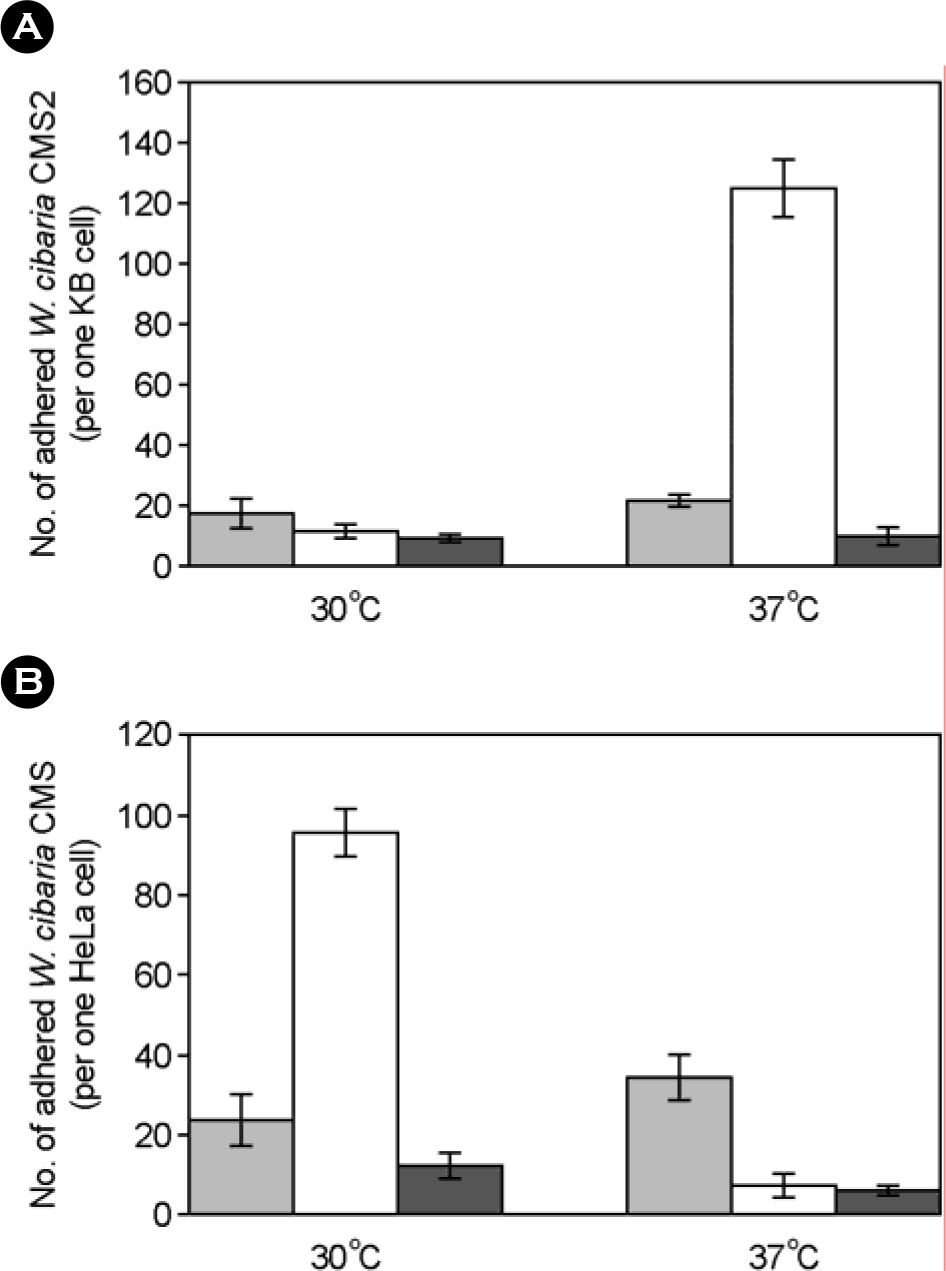
- We evaluated the ability of lactic acid bacteria, Weissella cibaria, isolated from the oral cavity to adhere to epithelial cells. W. cibaria efficiently adhered to KB cells and HeLa cells. In addition, W. cibaria efficiently adhered to Fusobacterium nucleatum. But the adhesiveness of W. cibaria disappeared upon exposure to LiCl or pronase, suggesting that the S-layer proteins of W. cibaria mediated the adhesiveness. The molecular mass of the S-layer proteins extracted from W. cibaria was approximately 50 kDa. When W. cibaria strains were washed with 0.45% saline, the bacteria were efficiently adhered to the epithelial cells. In conclusion, W. cibaria has the ability to adhere to epithelial cells through the S-layer proteins.
Keyword
MeSH Terms
Figure
Reference
-
References
1). Ahola AJ, Yli-Knuuttila H, Suomalainen T, Poussa T, Ahlström A, Meurman JH, Korpela R. Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch Oral Biol. 47:799–804. 2002.
Article2). Ahrne S, Nobaek S, Jeppsson B, Adlerberth I, Wold AE, Molin G. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J Appl Microbiol. 85:88–94. 1998.3). Bjorkroth KJ, Schillinger U, Geisen R, Weiss N, Hoste B, Holzapfel WH, Korkeala HJ, Vandamme P. Taxonomic study of Weissella confusa and description of Weissella cibaria sp. nov., detected in food and clinical samples. Int J Syst Evol Microbiol. 52:141–148. 2002.4). Boris S, Suárez JE, Barbés C. Characterization of the aggregation promoting factor from Lactobacillus gasseri, a vaginal isolate. J Appl Microbiol. 83:413–420. 1997.5). Choi HJ, Cheigh CI, Kim SB, Lee JC, Lee DW, Choi SW, Park JM, Pyun YR. Weissella kimchii sp. nov., a novel lactic acid bacterium from kimchi. Int J Syst Evol Microbiol. 52:507–511. 2002.6). De Vuyst L, Schrijvers V, Paramithiotis S, Hoste B, Vancanneyt M, Swings J, Kalantzopoulos G, Tsakalidou E, Messens W. The biodiversity of lactic acid bacteria in Greek traditional wheat sourdoughs is reflected in both composition and metabolite formation. Appl Environ Microbiol. 68:6059–6069. 2002.
Article7). Del Re B, Sgorbati B, Miglioli M, Palenzona D. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol. 31:438–442. 2000.8). Drago L, Gismondo MR, Lombardi A, de Haen C, Gozzini L. Inhibition of in vitro growth of enteropathogens by new Lactobacillus isolates of human intestinal origin. FEMS Microbiol Lett. 153:455–463. 1997.9). Fuller R. Probiotics in human medicine. Gut. 32:439–442. 1991.
Article10). Gill HS, Shu Q, Lin H, Rutherfurd KJ, Cross ML. Protection against translocating Salmonella typhimurium infection in mice by feeding the immuno-enhancing probiotic Lactobacillus rhamnosus strain HN001. Med Microbiol Immunol. 190:97–104. 2001.11). Gilliland SE, Nelson CR, Maxwell C. Assimilation of cholesterol by Lactobacillus acidophilus. Appl Environ Microbiol. 49:377–381. 1985.12). Kang MS, Chung J, Kim SM, Yang KH, Oh JS. Effect of Weissella cibaria isolates on the formation of Streptococcus mutans biofilm. Caries Res. 2006. (In press).13). Kang MS, Kim BG, Chung J, Lee HC, Oh JS. Inhibitory effect of Weissella cibaria isolates on the production of volatile sulfur compounds. J Clin Periodon. 33:226–232. 2006.14). Kang MS, Na HS, Oh JS. Coaggregation ability of Weissella cibaria isolates with Fusobacterium nucleatum and their adhesiveness to epithelial cells. FEMS Microbiol Lett. 253:323–329. 2005.15). Kinder SA, Holt SC. Coaggregation between bacterial species. Methods Enzymol. 236:254–270. 1994.
Article16). Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 54:413–437. 2000.
Article17). Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227:680–685. 1970.
Article18). Lamont RJ, Jenkinson HF. Adhesion as an ecological determinant in the oral cavity. pp. p. 131–168. In. Oral bacterial ecology: the molecular basis. Kuramitsu HK, Ellen RP, editors. (Ed),. Horizon Scientific Press;Wymondham: 2000.19). Lortal S, van Heijenoort J, Gruber K, Sleytr UB. S-layer of Lactobacillus helveticus ATCC 12046: isolation, chemical characterization and reformation after extraction with lithium chloride. J Gen Microbiol. 138:611–618. 1992.20). Messner P, Allmaier G, Schäffer C, Wugeditsch T, Lortal S, König H, Niemetz R, Dorner M. Biochemistry of S-layers. FEMS Microbiol Rev. 20:25–46. 1997.21). Redondo-López V, Cook RL, Sobel JD. Emerging role of lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 12:856–872. 1990.22). Reid G, McGroarty JA, Angotti R, Cook RL. Lactobacillus inhibitor production against Escherichia coli and coaggregation ability with uropathogens. Can J Microbiol. 34:344–351. 1988.23). Santos EM, Jaime I, Rovira J, Lyhs U, Korkeala H, Bjorkroth J. Characterization and identification of lactic acid bacteria in “morcilla de Burgos”. Int J Food Microbiol. 97:285–296. 2005.
Article24). Scaletsky IC, Silva ML, Trabulsi LR. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 45:534–536. 1984.25). Schneitz C, Nuotio L, Lounatma K. Adhesion of Lactobacillus acidophilus to avian intestinal epithelial cells mediated by the crystalline bacterial cell surface layer (S-layer). J Appl Bacteriol. 74:290–294. 1993.26). Stiles ME, Holzapfel WH. Lactic acid bacteria of foods and their current taxonomy. Int J Food Microbiol. 36:1–29. 1997.
Article27). Yasui T, Yoda K, Kamiya T. Analysis of S-layer proteins of Lactobacillus brevis. FEMS Microbiol Lett. 133:181–186. 1995.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Weissella cibaria on Fusobacterium nucleatum-induced Interleukin-6 and Interleukin-8 Production in KB Cells
- Comparison of Temperature and Additives Affecting the Stability of the Probiotic Weissella cibaria
- Immunomodulatory effects mixed with Weissella cibaria JW15 and Black soybean (Glycine max (L.) Merr.) Extract
- Quantitative Analysis of Weissella cibaria against Periodontopathic Bacteria by Real-time PCR
- Oral malodor-reducing effects by oral feeding of Weissella cibaria CMU in Beagle dogs