Int J Stem Cells.
2014 May;7(1):33-42.
Histolgical AND Immunohistochemical Study on the Effect of Stem Cell Therapy on Bleomycin Induced Pulmonary Fibrosis in Albino Rat
- Affiliations
-
- 1Department of Histology, Faculty of Medicine, Cairo University, Cairo, Egypt. marwasabry2020@yahoo.com
Abstract
- AIM OF WORK: To demonstrate the bleomycin induced histological changes in the lung and the possible protective and/or therapeutic effect of stem cell therapy.
MATERIALS AND METHODS
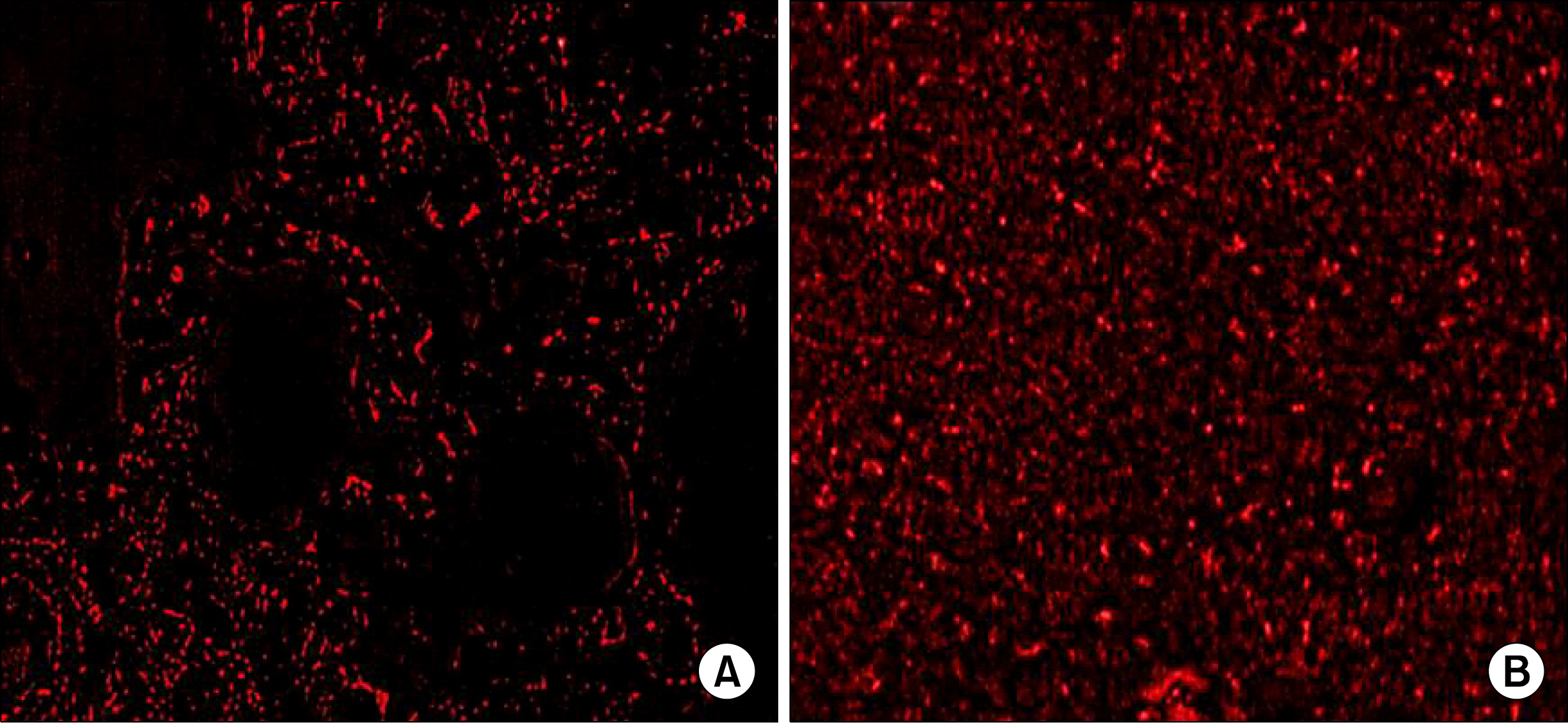
Study was carried out on 36 adult male albino rats, classified into 4 groups: group I (control), group II (bleomycin treated group), group III (early stem cell treated group: immediately after bleomycin), group IV (late stem cell treated group: 7 days after bleomycin). Sections were taken at the 14th day of experiment. stained with Hematoxylin and Eosin, Masson's trichrome, immunohistochemichal stains for alpha-SMA & PCNA. Sections were examined by light & immunofluroscent microscopy. Area percent of collagen fibers, area percent & optical density of alpha-SMA immunopositive cells were measured as well as the number of H&E and PCNA stained pneumocytes type II was counted.
RESULTS
Group II showed, thickening of septa, extravasation of blood, dividing pneumocytes type II cells with acinar formation, cellular infiltration, fibroblast cells, almost complete loss of normal lung architecture in certain fields, consolidation and replacement of the lung tissue with fibrous tissue in other fields. Restoring of lung tissue with significant decrease in mean area % of collagen fibers, alpha-SMA immunopositive cells were detected in group III.
CONCLUSIONS
Early treatment with bone marrow derived mesenchymal stem cells (BMSCs) immediately after bleomycin administration showed a significant reduction in fibrotic changes, however the late treatment with BMSCs (7 days) after bleomycin administration showed non significant results.
MeSH Terms
-
Adult
Animals
Bleomycin*
Bone Marrow
Collagen
Coloring Agents
Eosine Yellowish-(YS)
Fibroblasts
Hematoxylin
Humans
Lung
Male
Mesenchymal Stromal Cells
Microscopy
Pneumocytes
Proliferating Cell Nuclear Antigen
Pulmonary Fibrosis*
Rats*
Stem Cells*
Bleomycin
Collagen
Coloring Agents
Eosine Yellowish-(YS)
Hematoxylin
Proliferating Cell Nuclear Antigen
Figure
Reference
-
References
1. Keeley EC, Mehrad B, Strieter RM. Fibrocytes: bringing new insights into mechanisms of inflammation and fibrosis. Int J Biochem Cell Biol. 2010. 42:535–542.
Article2. Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011. 183:788–824.
Article3. Jindal SK. Interstitial lung disease. Jindal SK, editor. Textbook of Pulmonary and Critical Care Medicine. 1st ed. New Delhi; St Louis: JP Medical Ltd;2011. 1174–1188.4. Bugaut H, Bruchard M, Berger H, Derangère V, Odoul L, Euvrard R, Ladoire S, Chalmin F, Végran F, Rébé C, Apetoh L, Ghiringhelli F, Mignot G. Bleomycin exerts ambivalent antitumor immune effect by triggering both immunogenic cell death and proliferation of regulatory T cells. PLoS One. 2013. 8:e65181.
Article5. Zhao L, Wang X, Chang Q, Xu J, Huang Y, Guo Q, Zhang S, Wang W, Chen X, Wang J. Neferine, a bisbenzylisoquin-line alkaloid attenuates bleomycin-induced pulmonary fibrosis. Eur J Pharmacol. 2010. 627:304–312.
Article6. Kakugawa T, Mukae H, Hayashi T, Ishii H, Abe K, Fujii T, Oku H, Miyazaki M, Kadota J, Kohno S. Pirfenidone attenuates expression of HSP47 in murine bleomycin-induced pulmonary fibrosis. Eur Respir J. 2004. 24:57–65.
Article7. Hinz B. Regenerative Medicine and Biomaterials for the Repair of Connective Tissues. 2010. 39–82.8. Zhao R, Du L, Gunst SJ. Contractile and mechanical stimuli regulate cofilin phosphorylation (Cofilin-P) in tracheal smooth muscle (TSM) tissues. The FASEB Journal. 2006. 20:A1241.9. Pilling D, Tucker NM, Gomer RH. Aggregated IgG inhibits the differentiation of human fibrocytes. J Leukoc Biol. 2006. 79:1242–1251.
Article10. Zdunek M, Korobowicz E. Expression of PCNA in non-small cell lung cancer before and after treatment with cisplatin and vepeside. Pol J Pathol. 2000. 51:77–81.11. Scovassi AI, Prosperi E. Analysis of proliferating cell nuclear antigen (PCNA) associated with DNA. Methods Mol Biol. 2006. 314:457–475.12. Fukumoto J, Harada C, Kawaguchi T, Suetsugu S, Maeyama T, Inoshima I, Hamada N, Kuwano K, Nakanishi Y. Amphiregulin attenuates bleomycin-induced pneumopathy in mice. Am J Physiol Lung Cell Mol Physiol. 2010. 298:L131–L138.
Article13. Tzouvelekis A, Antoniadis A, Bouros D. Stem cell therapy in pulmonary fibrosis. Curr Opin Pulm Med. 2011. 17:368–373.
Article14. Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010. 1:19.
Article15. Rodman DM. Proof-of-concept trials for lung stem cell therapy. Proc Am Thorac Soc. 2008. 5:731–735.
Article16. Paget GE, And Barnes JM. Toxicty test. Laurnce DR, Bacharach AL, editors. Evaluation of drug activities: pharmacometrics. London: Academic press;1964. 135.17. Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A. 2003. 100:8407–8411.
Article18. Kiernan JA. Histological and histochemical methods: theory and practice. 3rd ed. London, New York & New Delhi: Arnold Publisher;2001. 111–162.19. Bancroft JD, Gamble M. Connective tissue stains. Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. 6th ed. Edinburgh, London, Oxford, New York, Philadelphia, St Louis, Sydney, Toronto: Elsevier Health Sciences, Churchill Livingstone;2008. 150.20. Bancroft JD, Cook HC. Immunocytochemistry. Bancroft JD, Cook HC, editors. Manual of histological techniques and their diagnostic applications. 2nd ed. London, Madrid, Melbourne, New York, Tokyo: Churchill Livingstone, Edinburgh;1994. 263–325.21. Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004. 13:436–448.
Article22. Rochefort GY, Vaudin P, Bonnet N, Pages JC, Domenech J, Charbord P, Eder V. Influence of hypoxia on the domiciliation of mesenchymal stem cells after infusion into rats: possibilities of targeting pulmonary artery remodeling via cells therapies? Respir Res. 2005. 6:125.
Article23. Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res. 2010. 19:237–270.
Article24. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003. 112:1776–1784.
Article25. Anversa P, Kajstura J, Leri A, Loscalzo J. Tissue-specific adult stem cells in the human lung. Nat Med. 2011. 17:1038–1039.
Article26. Condeelis J, Segall JE. Intravital imaging of cell movement in tumours. Nat Rev Cancer. 2003. 3:921–930.
Article27. Kulkarni AA, Thatcher TH, Hsiao HM, Olsen KC, Kottmann RM, Morrissette J, Wright TW, Phipps RP, Sime PJ. The triterpenoid CDDO-Me inhibits bleomycin-induced lung inflammation and fibrosis. PLoS One. 2013. 8:e63798.
Article28. Porada CD, Zanjani ED, Almeida-Porad G. Adult mesenchymal stem cells: a pluripotent population with multiple applications. Curr Stem Cell Res Ther. 2006. 1:365–369.
Article29. Fang X, Neyrinck AP, Matthay MA, Lee JW. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010. 285:26211–26222.
Article30. Cui A, Dai HP, Dai JW, Pang BS, Niu SJ, Lü YP, Wang C. Effects of bone marrow mesenchymal stem cells on bleomycin induced pulmonary fibrosis in rats. Zhonghua Jie He He Hu Xi Za Zhi. 2007. 30:677–682.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of nuclear factor-kappa B in bleomycin induced pulmonary fibrosis and the probable alleviating role of ginsenoside: histological, immunohistochemical, and biochemical study
- Inhibitory effect of nordihydroguiaretic acid on bleomycin-induced pulmonary fibrosis in rat
- Histological and Physiological Studies of the Effect of Bone Marrow-Derived Mesenchymal Stem Cells on Bleomycin Induced Lung Fibrosis in Adult Albino Rats
- A Case of Bleomycin Induced Bronchiolitis Obliterans Orgnizing Pneumonia
- The Effect of Steroid on the Bleomycin-Induced Pulmonary Fibrosis in Rat -Analysis of Cell Patterns in the Broncholaveolar Lavage Fluid, Histopathologic Findings and Total Collagen Content of the Lung-