Lab Anim Res.
2011 Jun;27(2):171-176. 10.5625/lar.2011.27.2.171.
Bone Marrow-Derived Mesenchymal Stem Cells Improve the Functioning of Neurotrophic Factors in a Mouse Model of Diabetic Neuropathy
- Affiliations
-
- 1Stem Cell Neuroplasticity Research Group, Kyungpook National University, Daegu, Republic of Korea.
- 2Department of Physiology, Cell and Matrix Research Institute, BSEI, World Class University Program, School of Medicine, Kyungpook National University, Daegu, Republic of Korea.
- 3Department of Laboratory Animal Medicine, Cell and Matrix Research Institute, College of Veterinary Medicine, Kyungpook National University, Daegu, Republic of Korea.
- KMID: 2053667
- DOI: http://doi.org/10.5625/lar.2011.27.2.171
Abstract
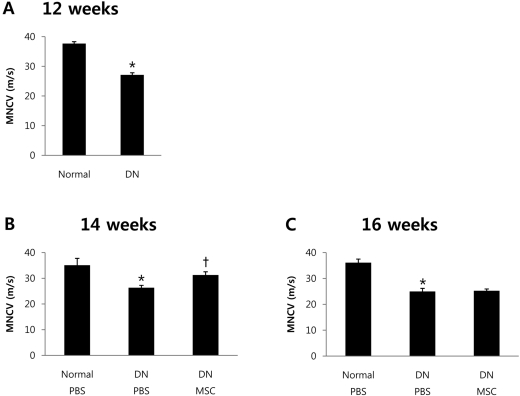
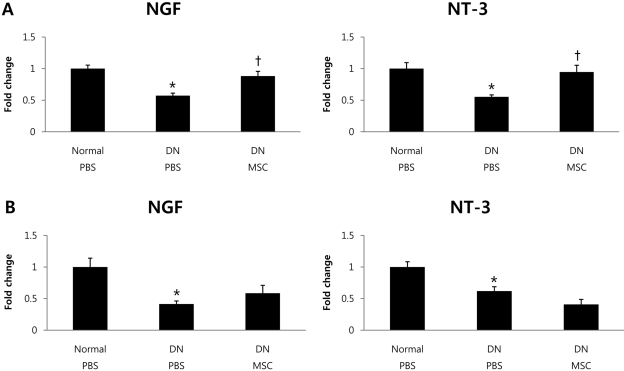
- Diabetic neuropathy is one of the most frequent and troublesome complications of diabetes. Although there has been a continuous increase in the incidence of diabetic neuropathy, treatments have yet to be found that effectively treat diabetic neuropathy. Neurotrophic factors are proteins that promote the survival of specific neuronal populations. They also play key roles in the regeneration of peripheral nervous system. Recent evidence from diabetic animal models and human diabetic subjects suggest that reduced availability of neurotrophic factors may contribute to the pathogenesis of diabetic neuropathy. One way to reverse this effect is to take advantage of the finding that bone marrow derived mesenchymal stem cells (BM-MSCs) promote peripheral nerve repair and the functioning of neurotrophic factors. Therefore, we speculated that treatment with BM-MSCs could be a viable therapeutic strategy for diabetic neuropathy. The present study was designed to examine the possible beneficial effect of BM-MSCs on functions of neurotrophic factors in diabetic neuropathy. To assess this possibility, we used an in vivo streptozotocin-induced diabetic neuropathy mouse model. Quantitative real-time polymerase-chain reacion showed that BM-MSCs significantly increase expression levels of neurotrophic factors. Also, BM-MSCs ameliorated nerve conduction velocity in streptozotocin-treated mice. These results may help to elucidate the mechanism by which BM-MSCs function as a cell therapy agent in diabetic neuropathy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Stem cell therapy in pain medicine
Yong Hee Han, Kyung Hoon Kim, Salahadin Abdi, Tae Kyun Kim
Korean J Pain. 2019;32(4):245-255. doi: 10.3344/kjp.2019.32.4.245.
Reference
-
1. Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med. 1980; 49(193):95–108. PMID: 7433630.2. Sampson MJ, Wilson S, Karagiannis P, Edmonds M, Watkins PJ. Progression of diabetic autonomic neuropathy over a decade in insulin-dependent diabetics. Q J Med. 1990; 75(278):635–646. PMID: 2217668.3. Vinik AI, Park TS, Stansberry KB, Pittenger GL. Diabetic neuropathies. Diabetologia. 2000; 43(8):957–973. PMID: 10990072.
Article4. Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009; 1792(10):931–940. PMID: 19061951.
Article5. Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003; 27(3):277–324. PMID: 12845152.
Article6. Apfel SC. Neurotrophic factors and diabetic peripheral neuropathy. Eur Neurol. 1999; 41(Suppl 1):27–34. PMID: 10023126.
Article7. Yano H, Chao MV. Neurotrophin receptor structure and interactions. Pharm Acta Helv. 2000; 74(2-3):253–260. PMID: 10812966.
Article8. Rodríguez-Tébar A, Dechant G, Götz R, Barde YA. Binding of neurotrophin-3 to its neuronal receptors and interactions with nerve growth factor and brain-derived neurotrophic factor. EMBO J. 1992; 11(3):917–922. PMID: 1547788.
Article9. Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk protooncogene product induced by NGF. Nature. 1991; 350(6314):158–160. PMID: 1706478.
Article10. Lamballe F, Klein R, Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991; 66(5):967–979. PMID: 1653651.
Article11. Chan JR, Cosgaya JM, Wu YJ, Shooter EM. Neurotrophins are key mediators of the myelination program in the peripheral nervous system. Proc Natl Acad Sci USA. 2001; 98(25):14661–14668. PMID: 11717413.
Article12. Chan JR, Watkins TA, Cosgaya JM, Zhang C, Chen L, Reichardt LF, Shooter EM, Barres BA. NGF controls axonal receptivity to myelination by Schwann cells or oligodendrocytes. Neuron. 2004; 43(2):183–191. PMID: 15260955.
Article13. Xiao J, Wong AW, Willingham MM, Kaasinen SK, Hendry IA, Howitt J, Putz U, Barrett GL, Kilpatrick TJ, Murray SS. BDNF exerts contrasting effects on peripheral myelination of NGF-dependent and BDNF-dependent DRG neurons. J Neurosci. 2009; 29(13):4016–4022. PMID: 19339597.
Article14. Tomlinson DR, Fernyhough P, Diemel LT. Neurotrophins and peripheral neuropathy. Philos Trans R Soc Lond B Biol Sci. 1996; 351(1338):455–462. PMID: 8730785.15. Tomlinson DR, Fernyhough P, Diemel LT. Role of neurotrophins in diabetic neuropathy and treatment with nerve growth factors. Diabetes. 1997; 46(Suppl 2):S43–S49. PMID: 9285498.
Article16. Kamiya H, Zhang W, Sima AA. C-peptide prevents nociceptive sensory neuropathy in type 1 diabetes. Ann Neurol. 2004; 56(6):827–835. PMID: 15497155.
Article17. Wang J, Ding F, Gu Y, Liu J, Gu X. Bone marrow mesenchymal stem cells promote cell proliferation and neurotrophic function of Schwann cells in vitro and in vivo. Brain Res. 2009; 1262:7–15. PMID: 19368814.
Article18. Cuevas P, Carceller F, Dujovny M, Garcia-Gómez I, Cuevas B, González-Corrochano R, Diaz-González D, Reimers D. Peripheral nerve regeneration by bone marrow stromal cells. Neurol Res. 2002; 24(7):634–638. PMID: 12392196.
Article19. Mimura T, Dezawa M, Kanno H, Sawada H, Yamamoto I. Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J Neurosurg. 2004; 101(5):806–812. PMID: 15540919.
Article20. Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998; 4(9):1025–1031. PMID: 9734395.
Article21. Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer's disease mice by modulation of immune responses. Stem Cells. 2010; 28(2):329–343. PMID: 20014009.
Article22. Jeong JO, Kim MO, Kim H, Lee MY, Kim SW, Ii M, Lee JU, Lee J, Choi YJ, Cho HJ, Lee N, Silver M, Wecker A, Kim DW, Yoon YS. Dual angiogenic and neurotrophic effects of bone marrow-derived endothelial progenitor cells on diabetic neuropathy. Circulation. 2009; 119(5):699–708. PMID: 19171856.
Article23. Fernyhough P, Diemel LT, Hardy J, Brewster WJ, Mohiuddin L, Tomlinson DR. Human recombinant nerve growth factor replaces deficient neurotrophic support in the diabetic rat. Eur J Neurosci. 1995; 7(5):1107–1110. PMID: 7613616.
Article24. Fernyhough P, Diemel LT, Tomlinson DR. Target tissue production and axonal transport of neurotrophin-3 are reduced in streptozotocin-diabetic rats. Diabetologia. 1998; 41(3):300–306. PMID: 9541170.
Article25. Christianson JA, Ryals JM, McCarson KE, Wright DE. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. J Pain. 2003; 4(9):493–504. PMID: 14636817.
Article26. Christianson JA, Ryals JM, Johnson MS, Dobrowsky RT, Wright DE. Neurotrophic modulation of myelinated cutaneous innervation and mechanical sensory loss in diabetic mice. Neuroscience. 2007; 145(1):303–313. PMID: 17223273.
Article27. Hellweg R, Hartung HD. Endogenous levels of nerve growth factor (NGF) are altered in experimental diabetes mellitus: a possible role for NGF in the pathogenesis of diabetic neuropathy. J Neurosci Res. 1990; 26(2):258–267. PMID: 2142224.
Article28. Hellweg R, Wöhrle M, Hartung HD, Stracke H, Hock C, Federlin K. Diabetes mellitus-associated decrease in nerve growth factor levels is reversed by allogeneic pancreatic islet transplantation. Neurosci Lett. 1991; 125(1):1–4. PMID: 1857552.
Article29. Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004; 10(6):610–616. PMID: 15156204.
Article30. Frostick SP, Yin Q, Kemp GJ. Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery. 1998; 18(7):397–405. PMID: 9880154.
Article31. Caddick J, Kingham PJ, Gardiner NJ, Wiberg M, Terenghi G. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia. 2006; 54(8):840–849. PMID: 16977603.
Article32. Brohlin M, Mahay D, Novikov LN, Terenghi G, Wiberg M, Shawcross SG, Novikova LN. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci Res. 2009; 64(1):41–49. PMID: 19428682.
Article33. Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci USA. 2005; 102(50):18171–18176. PMID: 16330757.
Article34. Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007; 354(3):700–706. PMID: 17257581.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cell Therapy for Diabetic Neuropathy Using Adult Stem or Progenitor Cells
- Neural Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells: Applicability for Inner Ear Therapy
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Bone Marrow-Derived Mesenchymal Stem Cells for Regenerative Medicine