Lab Anim Res.
2011 Jun;27(2):133-140. 10.5625/lar.2011.27.2.133.
Granulocyte-Derived Cationic Peptide Enhances Homing and Engraftment of Bone Marrow Stem Cells after Transplantation
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea. kspark@snu.ac.kr
- 2Innovative Research Institute for Cell Therapy, Seoul National University Hospital, Seoul, Republic of Korea.
- 3Biomedical Research Institute, Seoul National University Hospital, Seoul, Republic of Korea.
- KMID: 2053662
- DOI: http://doi.org/10.5625/lar.2011.27.2.133
Abstract
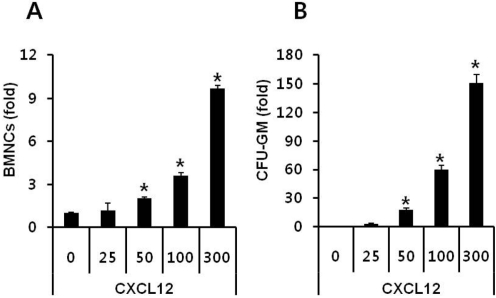
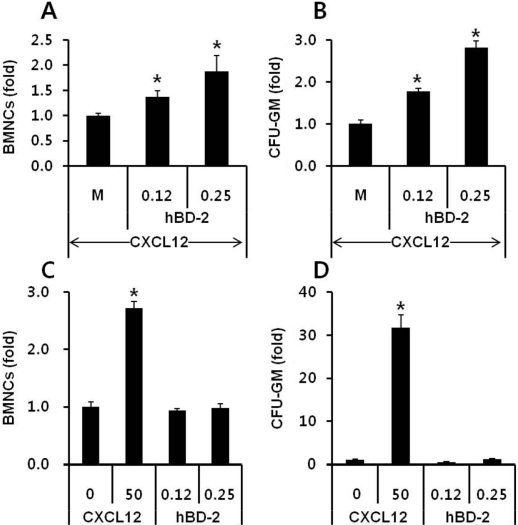
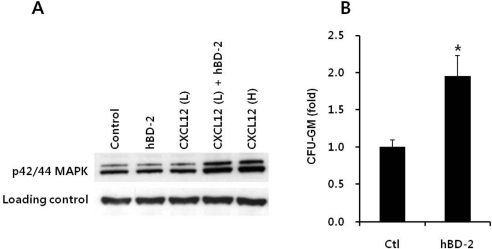
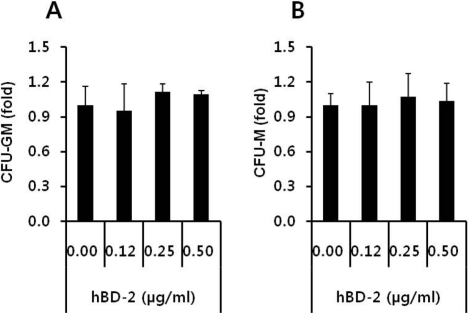
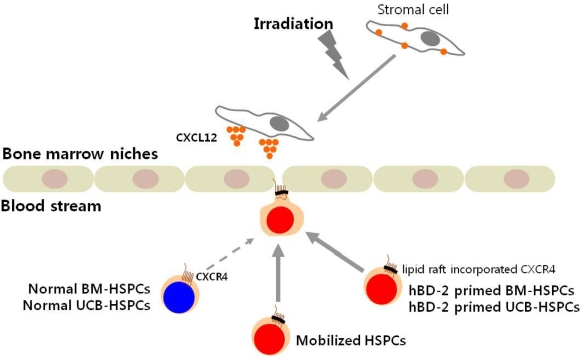
- Current strategies to accelerate hematopoietic reconstitution after transplantation include transplantation of greater numbers of hematopoietic stem/progenitor cells (HSPCs) or ex vivo expansion of harvested HSPCs before transplant. However, the number of cells available for transplantation is usually low, and strategies to expand HSPCs and maintain equivalent engraftment capability ex vivo are limited. We noted that activated granulocyte-derived cationic peptides positively primed responsiveness of HSPCs to a CXCL12 gradient. Accordingly, we noted that accelerated homing/engraftment of beta-defensin-2, a well-known antimicrobial cationic peptide, primed bone marrow nucleated cells (BMNCs) compared to normal BMNCs after transplantation into lethally irradiated recipients. We envision that small cationic peptides, which primarily possess antimicrobial functions and are harmless to mammalian cells, could be applied to prime HSPCs before transplantation. This novel approach would be particularly important in cord blood transplantation, where the number of HSPCs available for transplantation is usually limited.
Keyword
Figure
Cited by 1 articles
-
Bone marrow stem/progenitor cell mobilization in C57BL/6J and BALB/c mice
Hakmo Lee, Jeong-Hwan Che, Ju Eun Oh, Sung Soo Chung, Hye Seung Jung, Kyong Soo Park
Lab Anim Res. 2014;30(1):14-20. doi: 10.5625/lar.2014.30.1.14.
Reference
-
1. Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998; 92(2):362–367. PMID: 9657732.
Article2. Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003; 9(6):702–712. PMID: 12778169.
Article3. Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003; 101(10):4201–4208. PMID: 12560238.
Article4. Cogle CR, Yachnis AT, Laywell ED, Zander DS, Wingard JR, Steindler DA, Scott EW. Bone marrow transdifferentiation in brain after transplantation: a retrospective study. Lancet. 2004; 363(9419):1432–1427. PMID: 15121406.
Article5. Kassirer M, Zeltser D, Gluzman B, Leibovitz E, Goldberg Y, Roth A, Keren G, Rotstein R, Shapira I, Arber N, Berliner AS. The appearance of L-selectin(low) polymorphonuclear leukocytes in the circulating pool of peripheral blood during myocardial infarction correlates with neutrophilia and with the size of the infarct. Clin Cardiol. 1999; 22(11):721–726. PMID: 10554687.6. Kyne L, Hausdorff JM, Knight E, Dukas L, Azhar G, Wei JY. Neutrophilia and congestive heart failure after acute myocardial infarction. Am Heart J. 2000; 139(1 Pt 1):94–100. PMID: 10618568.
Article7. Matsunaga T, Sakamaki S, Kohgo Y, Ohi S, Hirayama Y, Niitsu Y. Recombinant human granulocyte colony-stimulating factor can mobilize sufficient amounts of peripheral blood stem cells in healthy volunteers for allogeneic transplantation. Bone Marrow Transplant. 1993; 11(2):103–108. PMID: 7679596.8. Sato N, Sawada K, Takahashi TA, Mogi Y, Asano S, Koike T, Sekiguchi S. A time course study for optimal harvest of peripheral blood progenitor cells by granulocyte colony-stimulating factor in healthy volunteers. Exp Hematol. 1994; 22(10):973–978. PMID: 7522184.9. Papayannopoulou T, Nakamoto B, Andrews RG, Lyman SD, Lee MY. In vivo effects of Flt3/Flk2 ligand on mobilization of hematopoietic progenitors in primates and potent synergistic enhancement with granulocyte colony-stimulating factor. Blood. 1997; 90(2):620–629. PMID: 9226162.10. Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005; 106(6):1901–1910. PMID: 15890683.
Article12. Devine SM, Lazarus HM, Emerson SG. Clinical application of hematopoietic progenitor cell expansion: current status and future prospects. Bone Marrow Transplant. 2003; 31(4):241–252. PMID: 12621458.
Article13. Papayannopoulou T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood. 2004; 103(5):1580–1585. PMID: 14604975.
Article14. Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998; 393(6685):595–599. PMID: 9634238.
Article15. Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, Rafii S. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci. 2001; 938:36–45. PMID: 11458524.
Article16. Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002; 16(10):1992–2003. PMID: 12357350.
Article17. Kushida T, Inaba M, Hisha H, Ichioka N, Esumi T, Ogawa R, Iida H, Ikehara S. Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood. 2001; 97(10):3292–3299. PMID: 11342461.
Article18. Wang J, Kimura T, Asada R, Harada S, Yokota S, Kawamoto Y, Fujimura Y, Tsuji T, Ikehara S, Sonoda Y. SCID-repopulating cell activity of human cord blood-derived CD34- cells assured by intra-bone marrow injection. Blood. 2003; 101(8):2924–2931. PMID: 12480697.
Article19. Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutsch V, Gazit Z, Gazit D, Karlsson S, Lapidot T. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004; 103(8):2942–2949. PMID: 15070669.
Article20. Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1-CXCR4 axis by the third complement component (C3)-Implications for trafficking of CXCR4+ stem cells. Exp Hematol. 2006; 34(8):986–995. PMID: 16863905.
Article21. Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, Glodek A, Honczarenko M, Spruce LA, Janowska-Wieczorek A, Lambris JD, Ratajczak MZ. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003; 101(10):3784–3793. PMID: 12511407.
Article22. Ratajczak MZ, Reca R, Wysoczynski M, Kucia M, Baran JT, Allendorf DJ, Ratajczak J, Ross GD. Transplantation studies in C3-deficient animals reveal a novel role of the third complement component (C3) in engraftment of bone marrow cells. Leukemia. 2004; 18(9):1482–1490. PMID: 15284858.
Article23. Malmsten M, Schmidtchen A. Antimicrobial C3a--biology, biophysics, and evolution. Adv Exp Med Biol. 2007; 598:141–158. PMID: 17892210.24. Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003; 3(9):710–720. PMID: 12949495.
Article25. Lee HM, Wysoczynski M, Liu R, Shin DM, Kucia M, Botto M, Ratajczak J, Ratajczak MZ. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2010; 24(3):573–582. PMID: 20033053.
Article26. Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, Kucia M, Janowska-Wieczorek A, Ratajczak J. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010; 24(5):976–985. PMID: 20357827.
Article27. Quesenberry PJ, Becker PS. Stem cell homing: rolling, crawling, and nesting. Proc Natl Acad Sci USA. 1998; 95(26):15155–15157. PMID: 9860935.
Article28. Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia. 2002; 16(10):1992–2003. PMID: 12357350.
Article29. Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997; 185(1):111–120. PMID: 8996247.
Article30. Möhle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998; 91(12):4523–4530. PMID: 9616148.31. Yang FC, Atkinson SJ, Gu Y, Borneo JB, Roberts AW, Zheng Y, Pennington J, Williams DA. Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc Natl Acad Sci USA. 2001; 98(10):5614–5618. PMID: 11320224.
Article32. Nguyen DH, Taub D. CXCR4 function requires membrane cholesterol: implications for HIV infection. J Immunol. 2002; 168(8):4121–4126. PMID: 11937572.
Article33. Raulin J. Human immunodeficiency virus and host cell lipids. Interesting pathways in research for a new HIV therapy. Prog Lipid Res. 2002; 41(1):27–65. PMID: 11694268.
Article34. Gómez-Moutón C, Lacalle RA, Mira E, Jiménez-Baranda S, Barber DF, Carrera AC, Martínez-A C, Mañes S. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J Cell Biol. 2004; 164(5):759–768. PMID: 14981096.35. Fernandis AZ, Cherla RP, Ganju RK. Differential regulation of CXCR4-mediated T-cell chemotaxis and mitogen-activated protein kinase activation by the membrane tyrosine phosphatase, CD45. J Biol Chem. 2003; 278(11):9536–9543. PMID: 12519755.
Article36. Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, Glodek A, Honczarenko M, Spruce LA, Janowska-Wieczorek A, Lambris JD, Ratajczak MZ. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003; 101(10):3784–3793. PMID: 12511407.
Article37. Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, Harris CE, Lee AW, Prabhakar R, Atkinson SJ, Kwiatkowski DJ, Williams DA. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003; 302(5644):445–449. PMID: 14564009.
Article38. Filippi MD, Harris CE, Meller J, Gu Y, Zheng Y, Williams DA. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat Immunol. 2004; 5(7):744–751. PMID: 15170212.
Article39. Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005; 11(8):886–891. PMID: 16025125.
Article40. Kim MS, Lee CS, Hur J, Cho HJ, Jun SI, Kim TY, Lee SW, Suh JW, Park KW, Lee HY, Kang HJ, Lee DS, Koh GY, Nakagami H, Morishita R, Park YB, Kim HS. Priming with angiopoietin-1 augments the vasculogenic potential of the peripheral blood stem cells mobilized with granulocyte colony-stimulating factor through a novel Tie2/Ets-1 pathway. Circulation. 2009; 120(22):2240–2250. PMID: 19917886.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Fixed Dose of G-CSF on Mobilization and Engraftment of Peripheral Blood Stem Cells in High Dose Chemotherapy
- Homing-Associated Cell Adhesion Molecules and Cell Cycle Status on the Nucleated Cells in the Bone Marrow, Mobilized Peripheral Blood and Cord Blood
- Cotransplanted Bone Marrow Derived Mesenchymal Stem Cells (MSC) Enhanced Engraftment of Hematopoietic Stem Cells in a MSC-dose Dependent Manner in NOD/SCID Mice
- The role of mesenchymal stem cells in hematopoietic stem cell transplantation
- Bone marrow-derived stem cells contribute to regeneration of the endometrium