Tuberc Respir Dis.
2014 Nov;77(5):203-208. 10.4046/trd.2014.77.5.203.
Effects of Lobetyolin, Lobetyol and Methyl linoleate on Secretion, Production and Gene Expression of MUC5AC Mucin from Airway Epithelial Cells
- Affiliations
-
- 1Department of Pharmacology, Chungnam National University School of Medicine, Daejeon, Korea. LCJ123@cnu.ac.kr
- 2Department of Pharmacy, College of Pharmacy, Yeungnam University, Daegu, Korea.
- 3Department of Pharmacy, College of Pharmacy, Seoul National University, Seoul, Korea.
- 4Pulmonology Section, Department of Internal Medicine, Chungnam National University Hospital, Daejeon, Korea.
- KMID: 2050712
- DOI: http://doi.org/10.4046/trd.2014.77.5.203
Abstract
- BACKGROUND
In this study, we investigated whether lobetyolin, lobetyol, and methyl linoleate derived from Codonopsis pilosula affect MUC5AC mucin secretion, production, and gene expression from airway epithelial cells.
METHODS
Confluent NCI-H292 cells were pretreated with lobetyolin, lobetyol, or methyl linoleate for 30 minutes and then stimulated with phorbol 12-myristate 13-acetate (PMA) for 24 hours. The MUC5AC mucin gene expression, and mucin protein production and secretion were measured by reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay, respectively.
RESULTS
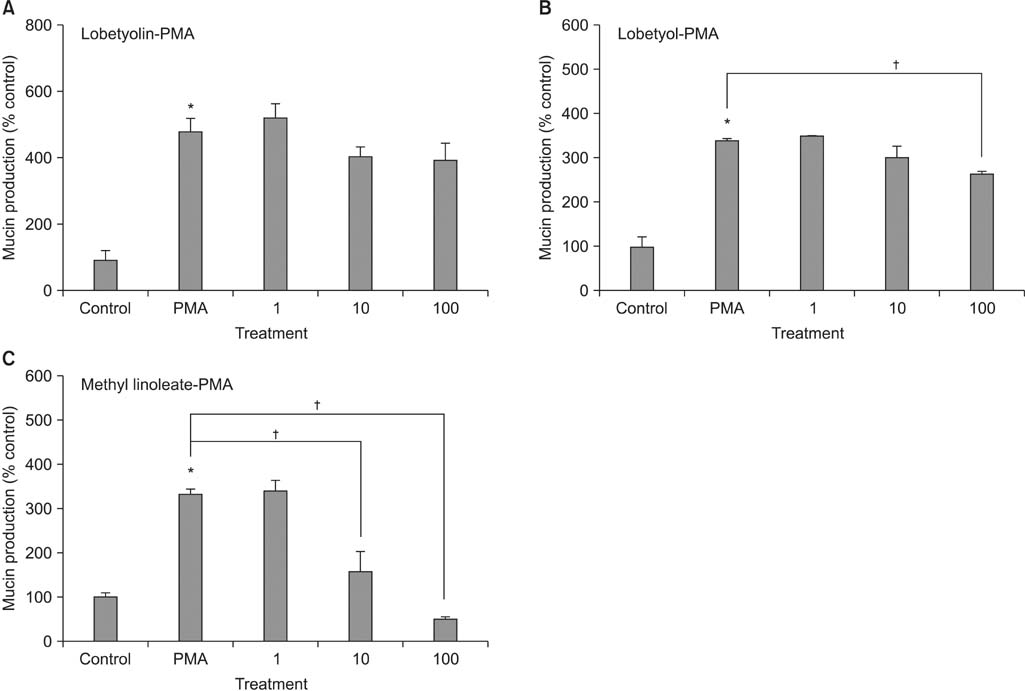
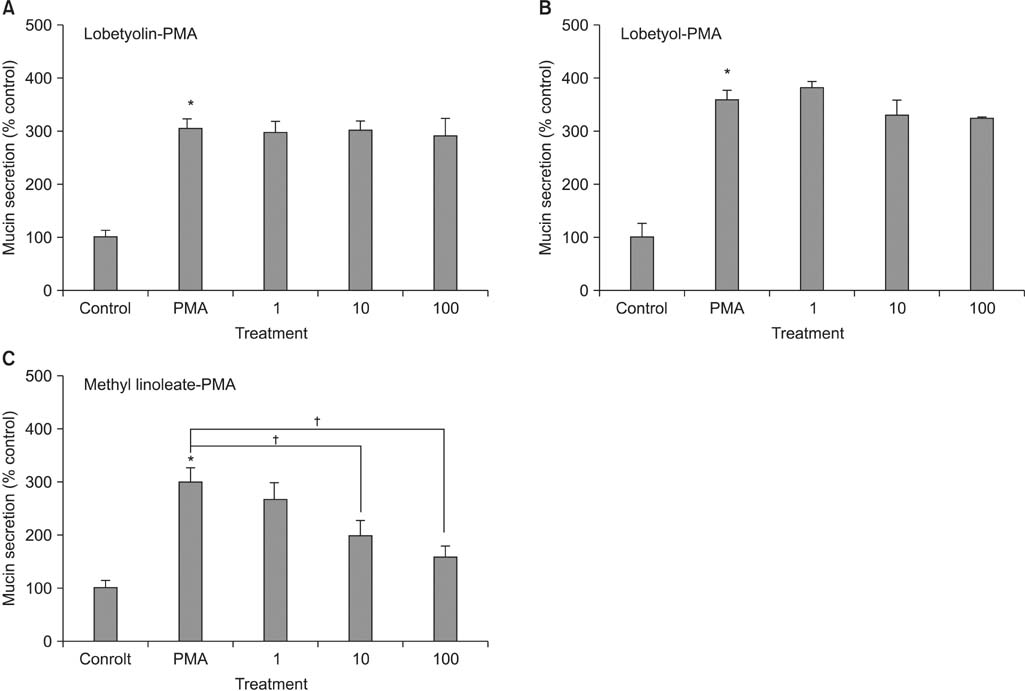
Lobetyolin, lobetyol, and methyl linoleate inhibited the gene expression of MUC5AC mucin induced by PMA; lobetyolin did not affect PMA-induced MUC5AC mucin production. However, lobetyol and methyl linoleate inhibited the production of MUC5AC mucin; lobetyolin and lobetyol did not significantly affect PMA-induced MUC5AC mucin secretion from NCI-H292 cells. However, methyl linoleate decreased the MUC5AC mucin secretion.
CONCLUSION
These results suggest that among the three compounds, methyl linoleate can regulate gene expression, production, and secretion of MUC5AC mucin by directly acting on the airway epithelial cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee CJ, Paik SH, Ko KH, Kim KC. Effects of polycationic peptides on mucin release from airway goblet cells: relationship between polymer size and activity. Inflamm Res. 2002; 51:490–494.2. Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009; 135:505–512.3. Heo HJ, Lee HJ, Kim YS, Kang SS, Son KH, Seok JH, et al. Effects of baicalin and wogonin on mucin release from cultured airway epithelial cells. Phytother Res. 2007; 21:1130–1134.4. Heo HJ, Lee SY, Lee MN, Lee HJ, Seok JH, Lee CJ. Genistein and curcumin suppress epidermal growth factor-induced MUC5AC mucin production and gene expression from human airway epithelial cells. Phytother Res. 2009; 23:1458–1461.5. Kim KD, Lee HJ, Lim SP, Sikder A, Lee SY, Lee CJ. Silibinin regulates gene expression, production and secretion of mucin from cultured airway epithelial cells. Phytother Res. 2012; 26:1301–1307.6. Jang IM. Treatise on Asian herbal medicines. Seoul: Haksul Pyunsukwan in Research Institute of Natural Products of Seoul National University;2003.7. Dumlu MU, Gurkan E, Tuzlaci E. Chemical composition and antioxidant activity of Campanula alliariifolia. Nat Prod Res. 2008; 22:477–482.8. Nand P, Drabu S, Gupta RK. Antimicrobial investigation of Linum usitatissimum for the treatment of acne. Nat Prod Commun. 2011; 6:1701–1704.9. Li JD, Dohrman AF, Gallup M, Miyata S, Gum JR, Kim YS, et al. Transcriptional activation of mucin by Pseudomonas aeruginosa lipopolysaccharide in the pathogenesis of cystic fibrosis lung disease. Proc Natl Acad Sci U S A. 1997; 94:967–972.10. Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, et al. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999; 96:3081–3086.11. Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2003; 100:11618–11623.12. Rogers DF, Barnes PJ. Treatment of airway mucus hypersecretion. Ann Med. 2006; 38:116–125.13. Hong DH, Petrovics G, Anderson WB, Forstner J, Forstner G. Induction of mucin gene expression in human colonic cell lines by PMA is dependent on PKC-epsilon. Am J Physiol. 1999; 277(5 Pt 1):G1041–G1047.14. Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol. 2004; 344:683–695.15. Park SJ, Kang SY, Kim NS, Kim HM. Phosphatidylinositol 3-kinase regulates PMA-induced differentiation and superoxide production in HL-60 cells. Immunopharmacol Immunotoxicol. 2002; 24:211–226.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Homogentisic Acid and Natural Products Derived from Pinellia ternata on Secretion, Production and Gene Expression of MUC5AC Mucin from Cultured Airway Epithelial Cells
- Effects of Cynaroside, Cynarin and Linarin on Secretion, Production and Gene Expression of Airway MUC5AC Mucin in NCI-H292 Cells
- Eriodictyol Inhibits the Production and Gene Expression of MUC5AC Mucin via the IκBα-NF-κB p65 Signaling Pathway in Airway Epithelial Cells
- Effect of Prostaglandin E2 on Gel-forming Mucin Secretion in Normal Human Nasal Epithelial Cells
- Effects of Nodakenin, Columbianadin, and Umbelliferone Isolated from the Roots of Angelica decursiva on the Gene Expression and Production of MUC5AC Mucin from Human Airway Epithelial NCI-H292 Cells