Clin Exp Vaccine Res.
2015 Jan;4(1):107-113. 10.7774/cevr.2015.4.1.107.
Oral immunization of mice with recombinant rabies vaccine strain (ERAG3G) induces complete protection
- Affiliations
-
- 1Viral Disease Division, Animal and Plant Quarantine Agency, MAFRA, Anyang, Korea. yangdk@korea.kr
- 2College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea.
- 3Gangwon-do Veterinary Service Laboratory, Chuncheon, Korea.
- KMID: 2049114
- DOI: http://doi.org/10.7774/cevr.2015.4.1.107
Abstract
- PURPOSE
New rabies vaccine bait for both pets and raccoon dogs residing in Korea is needed to eradicate rabies infection among animals. In this study, we constructed a recombinant rabies virus (RABV), the ERAG3G strain, using a reverse genetics system. Then we investigated the efficacy of this strain in mice after oral administration and the safety of this strain in cats after intramuscular administration.
MATERIALS AND METHODS
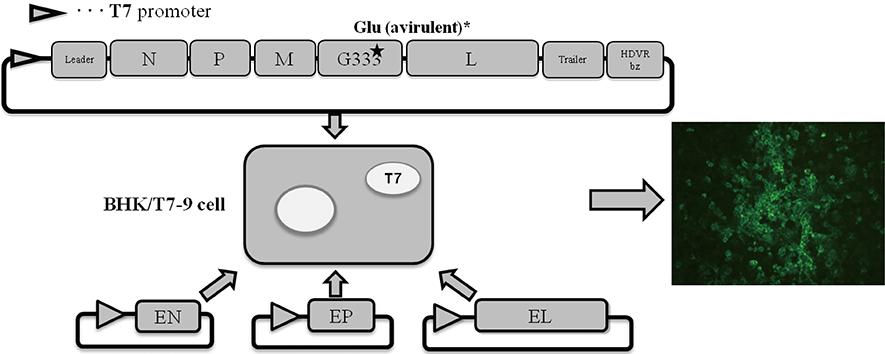
The ERAG3G strain was rescued in BHK/T7-9 cells using the full-length genome mutated at the amino acid position 333 of the glycoprotein gene of RABV and helper plasmids. Four-week-old mice underwent one or two oral administrations of the ERAG3G strain and were challenged with the highly virulent RABV strain CVSN2c 14 days after the second administration. Clinical symptoms were observed and body weights were measured every day after the challenge.
RESULTS
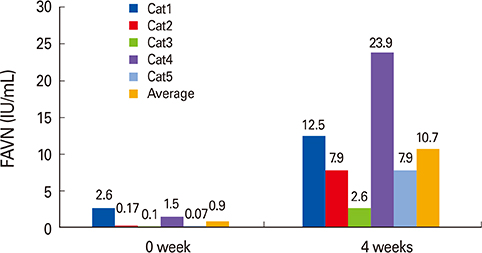
All mice showed complete protection against virulent RABV. In addition, cats intramuscularly inoculated with the ERAG3G strain showed high antibody titers ranging from 2.62 to 23.9 IU/mL at 28-day postinoculation.
CONCLUSION
The oral immunization of the ERAG3G strain plays an important role in conferring complete protection in mice, and intramuscular inoculation of the ERAG3G strain induces the formation of anti-rabies neutralizing antibody in cats.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Safety and Immunogenicity of a Recombinant Rabies Virus Strain (ERAG3G) in Korean Raccoon Dogs
Dong-Kun Yang, Ha-Hyun Kim, Hyun-Ye Jo, Hee-Won Kim, Sung-Suk Choi, In-Soo Cho
J Bacteriol Virol. 2015;45(3):250-255. doi: 10.4167/jbv.2015.45.3.250.
Reference
-
1. Rupprecht CE, Hanlon CA, Slate D. Oral vaccination of wildlife against rabies: opportunities and challenges in prevention and control. Dev Biol (Basel). 2004; 119:173–184.2. Yang DK, Kim SY, Oh YI, et al. Epidemiological characteristics of rabies in South Korea from January 2004 to March 2011. J Bacteriol Virol. 2011; 41:165–171.
Article3. Cliquet F, Gurbuxani JP, Pradhan HK, et al. The safety and efficacy of the oral rabies vaccine SAG2 in Indian stray dogs. Vaccine. 2007; 25:3409–3418.
Article4. Orciari LA, Niezgoda M, Hanlon CA, et al. Rapid clearance of SAG-2 rabies virus from dogs after oral vaccination. Vaccine. 2001; 19:4511–4518.
Article5. Fehlner-Gardiner C, Rudd R, Donovan D, Slate D, Kempf L, Badcock J. Comparing ONRAB(R) AND RABORAL V-RG(R) oral rabies vaccine field performance in raccoons and striped skunks, New Brunswick, Canada, and Maine, USA. J Wildl Dis. 2012; 48:157–167.
Article6. Taylor J, Meignier B, Tartaglia J, et al. Biological and immunogenic properties of a canarypox-rabies recombinant, ALVAC-RG (vCP65) in non-avian species. Vaccine. 1995; 13:539–549.
Article7. Yuan Z, Zhang S, Liu Y, et al. A recombinant pseudorabies virus expressing rabies virus glycoprotein: safety and immunogenicity in dogs. Vaccine. 2008; 26:1314–1321.
Article8. Slate D, Rupprecht CE, Rooney JA, Donovan D, Lein DH, Chipman RB. Status of oral rabies vaccination in wild carnivores in the United States. Virus Res. 2005; 111:68–76.
Article9. Joo YS, Lee JH, Lee KK, Bang HA, Lee WC. Retrospective study of extensive vaccination programs for canine rabies control and public health in Korea. Jpn J Infect Dis. 2011; 64:513–515.10. Rupprecht CE, Blass L, Smith K, et al. Human infection due to recombinant vaccinia-rabies glycoprotein virus. N Engl J Med. 2001; 345:582–586.
Article11. Li J, Faber M, Papaneri A, et al. A single immunization with a recombinant canine adenovirus expressing the rabies virus G protein confers protective immunity against rabies in mice. Virology. 2006; 356:147–154.
Article12. Franka R, Wu X, Jackson FR, et al. Rabies virus pathogenesis in relationship to intervention with inactivated and attenuated rabies vaccines. Vaccine. 2009; 27:7149–7155.
Article13. Mansfield KL, Johnson N, Nunez A, Hicks D, Jackson AC, Fooks AR. Up-regulation of chemokine gene transcripts and T-cell infiltration into the central nervous system and dorsal root ganglia are characteristics of experimental European bat lyssavirus type 2 infection of mice. J Neurovirol. 2008; 14:218–228.
Article14. Wang ZW, Sarmento L, Wang Y, et al. Attenuated rabies virus activates, while pathogenic rabies virus evades, the host innate immune responses in the central nervous system. J Virol. 2005; 79:12554–12565.
Article15. Srithayakumar V, Sribalachandran H, Rosatte R, Nadin-Davis SA, Kyle CJ. Innate immune responses in raccoons after raccoon rabies virus infection. J Gen Virol. 2014; 95:16–25.
Article16. Inoue K, Shoji Y, Kurane I, Iijima T, Sakai T, Morimoto K. An improved method for recovering rabies virus from cloned cDNA. J Virol Methods. 2003; 107:229–236.
Article17. Ito N, Takayama-Ito M, Yamada K, Hosokawa J, Sugiyama M, Minamoto N. Improved recovery of rabies virus from cloned cDNA using a vaccinia virus-free reverse genetics system. Microbiol Immunol. 2003; 47:613–617.
Article18. Yang DK, Nakagawa K, Ito N, et al. A single immunization with recombinant rabies virus (ERAG3G) confers complete protection against rabies in mice. Clin Exp Vaccine Res. 2014; 3:176–184.
Article19. Cliquet F, Aubert M, Sagne L. Development of a fluorescent antibody virus neutralisation test (FAVN test) for the quantitation of rabies-neutralising antibody. J Immunol Methods. 1998; 212:79–87.
Article20. Asokan GV, Asokan V, Tharyan P. One health national programme across species on zoonoses: a call to the developing world. Infect Ecol Epidemiol. 2011; 1:IEE-1-8293.
Article21. Fu ZF. The rabies situation in Far East Asia. Dev Biol (Basel). 2008; 131:55–61.22. Zhang S, Liu Y, Hou Y, et al. Epidemic and maintenance of rabies in Chinese ferret badgers (Melogale moschata) indicated by epidemiology and the molecular signatures of rabies viruses. Virol Sin. 2013; 28:146–151.
Article23. Sterner RT, Meltzer MI, Shwiff SA, Slate D. Tactics and economics of wildlife oral rabies vaccination, Canada and the United States. Emerg Infect Dis. 2009; 15:1176–1184.
Article24. Baer GM, Abelseth MK, Debbie JG. Oral vaccination of foxes against rabies. Am J Epidemiol. 1971; 93:487–490.
Article25. Nakagawa K, Ito N, Masatani T, et al. Generation of a live rabies vaccine strain attenuated by multiple mutations and evaluation of its safety and efficacy. Vaccine. 2012; 30:3610–3617.
Article26. Schnell MJ, Mebatsion T, Conzelmann KK. Infectious rabies viruses from cloned cDNA. Embo J. 1994; 13:4195–4203.
Article27. Takayama-Ito M, Inoue K, Shoji Y, et al. A highly attenuated rabies virus HEP-Flury strain reverts to virulent by single amino acid substitution to arginine at position 333 in glycoprotein. Virus Res. 2006; 119:208–215.
Article28. Faber M, Dietzschold B, Li J. Immunogenicity and safety of recombinant rabies viruses used for oral vaccination of stray dogs and wildlife. Zoonoses Public Health. 2009; 56:262–269.
Article29. Cliquet F, Aubert M. Elimination of terrestrial rabies in Western European countries. Dev Biol (Basel). 2004; 119:185–204.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A single immunization with recombinant rabies virus (ERAG3G) confers complete protection against rabies in mice
- Safety and Immunogenicity of a Recombinant Rabies Virus Strain (ERAG3G) in Korean Raccoon Dogs
- Safety and immunogenicity of recombinant rabies virus (ERAGS) in mice and raccoon dogs
- The present and future of rabies vaccine in animals
- Immunogenicity and efficacy of a plasmid DNA rabies vaccine incorporating Myd88 as a genetic adjuvant