Clin Exp Vaccine Res.
2015 Jan;4(1):88-98. 10.7774/cevr.2015.4.1.88.
Systemic antibody response to nano-size calcium phospate biocompatible adjuvant adsorbed HEV-71 killed vaccine
- Affiliations
-
- 1Department of Microbiology and Parasitology, University of Putra Malaysia, Medical Faculty, Serdang, Malaysia. zamberi@upm.edu.my
- 2University of Putra Malaysia, Institute of Biosciences, Serdang, Malaysia.
- 3University of Putra Malaysia, Institute of Advanced Technology, Serdang, Malaysia.
- 4Department of Microbiology and Parasitology, University of Khartoum, Faculty of Medicine, Sudan, Malaysia.
- KMID: 2049112
- DOI: http://doi.org/10.7774/cevr.2015.4.1.88
Abstract
- PURPOSE
Since 1980s, human enterovirus-71 virus (HEV-71) is one of the common infectious disease in Asian Pacific region since late 1970s without effective commercial antiviral or protective vaccine is unavailable yet. The work examines the role of vaccine adjuvant particle size and the route of administration on postvaccination antibody response towards HEV-71 vaccine adsorbed to calcium phosphate (CaP) adjuvant.
MATERIALS AND METHODS
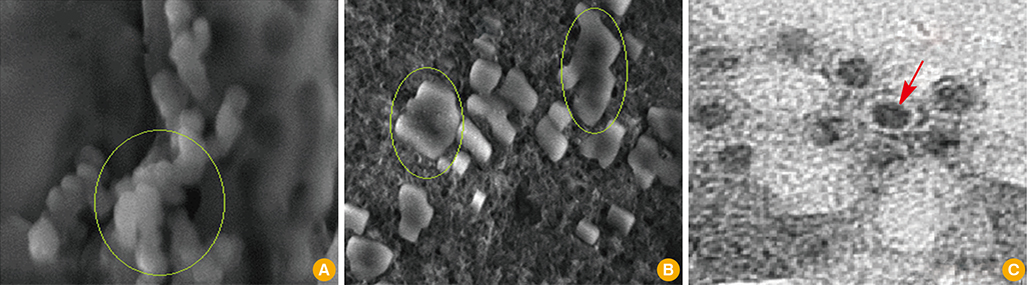
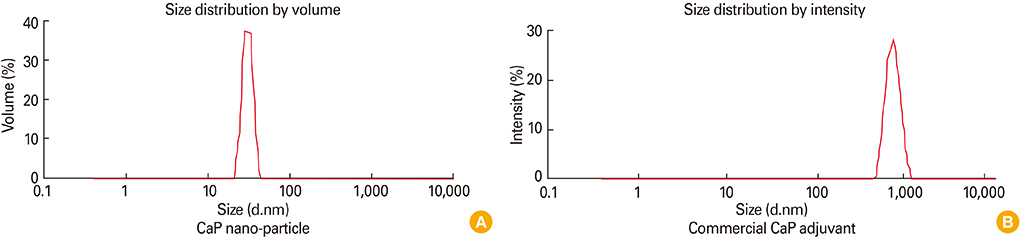
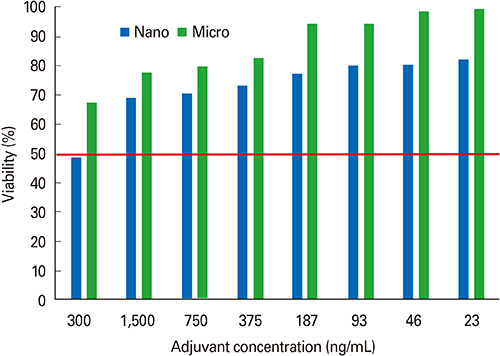
First, CaP nano-particles were compared to a commercial micro-size and vaccine alone. Secondly, intradermal reduced dosage was compared to the conventional intramuscular immunization. Killed HEV-71 vaccines adsorbed to CaP nano-size (73 nm) and commercial one of micro-size (1.7 microm) were administered through intradermal, intramuscular, rabbits received vaccine alone and unvaccinated animals.
RESULTS
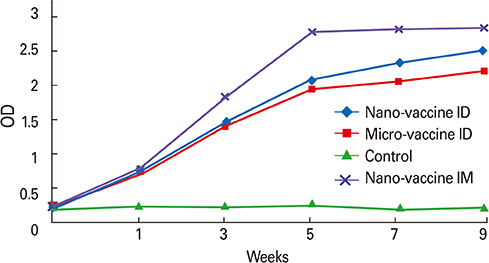
CaP nano-particles adsorbed HEV-71 vaccine displayed higher antibody than the micro-size or unadsorbed vaccine alone, through both parenteral immunization routes. Moreover, the intradermal route (0.5 microg/mL) of 0.1-mL volume per vaccine dose induced equal IgG antibody level to 1.0-mL intramuscular route (0.5 microg/mL).
CONCLUSION
The intradermal vaccine adsorbed CaP nano-adjuvant showed safer and significant antibody response after one-tenth reduced dose quantity (0.5 microg/mL) of only 0.1-mL volume as the most suitable protective, cost effective and affordable formulation not only for HEV-71; but also for developing further effective vaccines toward other human pathogens.
Keyword
MeSH Terms
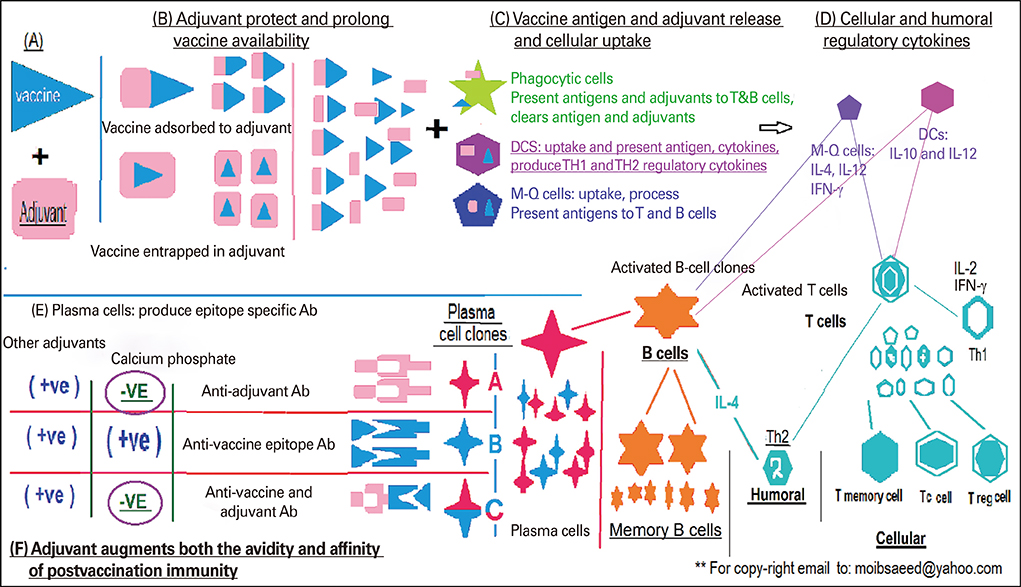
Figure
Reference
-
1. Chan YF, Sam IC, AbuBakar S. Phylogenetic designation of enterovirus 71 genotypes and subgenotypes using complete genome sequences. Infect Genet Evol. 2010; 10:404–412.
Article2. Boursnell ME, Entwisle C, Blakeley D, et al. A genetically inactivated herpes simplex virus type 2 (HSV-2) vaccine provides effective protection against primary and recurrent HSV-2 disease. J Infect Dis. 1997; 175:16–25.
Article3. Shi W, Li K, Ji Y, Jiang Q, Shi M, Mi Z. Development and evaluation of reverse transcription-loop-mediated isothermal amplification assay for rapid detection of enterovirus 71. BMC Infect Dis. 2011; 11:197.
Article4. Chang JY, Chang CP, Tsai HH, et al. Selection and characterization of vaccine strain for Enterovirus 71 vaccine development. Vaccine. 2012; 30:703–711.
Article5. De W, Changwen K, Wei L, et al. A large outbreak of hand, foot, and mouth disease caused by EV71 and CAV16 in Guangdong, China, 2009. Arch Virol. 2011; 156:945–953.
Article6. Lee MS, Tseng FC, Wang JR, Chi CY, Chong P, Su IJ. Challenges to licensure of enterovirus 71 vaccines. PLoS Negl Trop Dis. 2012; 6:e1737.
Article7. Yang C, Deng C, Wan J, Zhu L, Leng Q. Neutralizing antibody response in the patients with hand, foot and mouth disease to enterovirus 71 and its clinical implications. Virol J. 2011; 8:306.
Article8. Yan XF, Gao S, Xia JF, Ye R, Yu H, Long JE. Epidemic characteristics of hand, foot, and mouth disease in Shanghai from 2009 to 2010: enterovirus 71 subgenotype C4 as the primary causative agent and a high incidence of mixed infections with coxsackievirus A16. Scand J Infect Dis. 2012; 44:297–305.
Article9. Bek EJ, Hussain KM, Phuektes P, et al. Formalin-inactivated vaccine provokes cross-protective immunity in a mouse model of human enterovirus 71 infection. Vaccine. 2011; 29:4829–4838.
Article10. Huang SC, Hsu YW, Wang HC, et al. Appearance of intratypic recombination of enterovirus 71 in Taiwan from 2002 to 2005. Virus Res. 2008; 131:250–259.
Article11. Larsen GR, Anderson CW, Dorner AJ, Semler BL, Wimmer E. Cleavage sites within the poliovirus capsid protein precursors. J Virol. 1982; 41:340–344.
Article12. McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002; 26:91–107.
Article13. Craig ME, Howard NJ, Silink M, Rawlinson WD. Reduced frequency of HLA DRB1*03-DQB1*02 in children with type 1 diabetes associated with enterovirus RNA. J Infect Dis. 2003; 187:1562–1570.
Article14. Yang F, Ren L, Xiong Z, et al. Enterovirus 71 outbreak in the People's Republic of China in 2008. J Clin Microbiol. 2009; 47:2351–2352.
Article15. Huang SW, Hsu YW, Smith DJ, et al. Reemergence of enterovirus 71 in 2008 in taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008. J Clin Microbiol. 2009; 47:3653–3662.
Article16. Wong SS, Yip CC, Lau SK, Yuen KY. Human enterovirus 71 and hand, foot and mouth disease. Epidemiol Infect. 2010; 138:1071–1089.
Article17. Lee MS, Chang LY. Development of enterovirus 71 vaccines. Expert Rev Vaccines. 2010; 9:149–156.
Article18. Wu CN, Lin YC, Fann C, Liao NS, Shih SR, Ho MS. Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine. 2001; 20:895–904.
Article19. Singh M, Chakrapani A, O'Hagan D. Nanoparticles and microparticles as vaccine-delivery systems. Expert Rev Vaccines. 2007; 6:797–808.
Article20. Kiparissides C, Kammona O. Nanoscale carriers for targeted delivery of drugs and therapeutic biomolecules. Can J Chem Eng. 2013; 91:638–651.
Article21. Duclos P. Safety of immunisation and adverse events following vaccination against hepatitis B. Expert Opin Drug Saf. 2003; 2:225–231.
Article22. Chung YC, Ho MS, Wu JC, et al. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine. 2008; 26:1855–1862.
Article23. Liu CC, Chou AH, Lien SP, et al. Identification and characterization of a cross-neutralization epitope of enterovirus 71. Vaccine. 2011; 29:4362–4372.
Article24. van der Sanden S, van der Avoort H, Lemey P, Uslu G, Koopmans M. Evolutionary trajectory of the VP1 gene of human enterovirus 71 genogroup B and C viruses. J Gen Virol. 2010; 91:1949–1958.
Article25. Foo DG, Alonso S, Phoon MC, Ramachandran NP, Chow VT, Poh CL. Identification of neutralizing linear epitopes from the VP1 capsid protein of enterovirus 71 using synthetic peptides. Virus Res. 2007; 125:61–68.
Article26. Chen HF, Chang MH, Chiang BL, Jeng ST. Oral immunization of mice using transgenic tomato fruit expressing VP1 protein from enterovirus 71. Vaccine. 2006; 24:2944–2951.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extrahepatic manifestations of hepatitis E virus: An overview
- Protective effects and immunogenicity of Salmonella Enteritidis killed vaccine strains selected from virulent Salmonella Enteritidis isolates
- Outer membrane proteins of Salmonella typhimurium as an adjuvant in rabies vaccine
- Effects of Nano-sized Calcium on Intestinal Absorption and Bone Turnover
- Comparative evaluation of gold nanoparticles and Alum as immune enhancers against rabies vaccine and related immune reactivity, physiological, and histopathological alterations: in vivo study