Clin Exp Vaccine Res.
2015 Jan;4(1):75-82. 10.7774/cevr.2015.4.1.75.
Preliminary study on the immunogenicity of a newly developed GCC Tdap vaccine and its protection efficacy against Bordetella pertussis in a murine intranasal challenge model
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea. kjhan@catholic.ac.kr
- 2The Vaccine Bio Research Institute, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2049110
- DOI: http://doi.org/10.7774/cevr.2015.4.1.75
Abstract
- PURPOSE
Active reduced dose tetanus-diphtheria-acellular pertussis (Tdap) vaccination for adolescents and adults is necessary because waning immunity after primary diphtheria-tetanus-pertussis vaccination is related to the recent emergence of pertussis. This study was conducted to compare the immunogenicity and protection efficacy against Bordetella pertussis between a new GCC Tdap vaccine and a commercially available Tdap vaccine in a murine model.
MATERIALS AND METHODS
BALB/c mice were immunized with two doses of diphtheria-tetanus-acellular pertussis (DTaP) vaccine for priming and a subsequent Tdap booster vaccination. According to the type of booster vaccine, mice were divided into four groups: commercially available Tdap vaccine in group 1 and GCC Tdap vaccines of different combinations of pertussis antigens in groups 2 to 4. Humoral and cell-mediated immune responses and protection efficacy using a murine intranasal challenge model after booster vaccination were compared among the four groups.
RESULTS
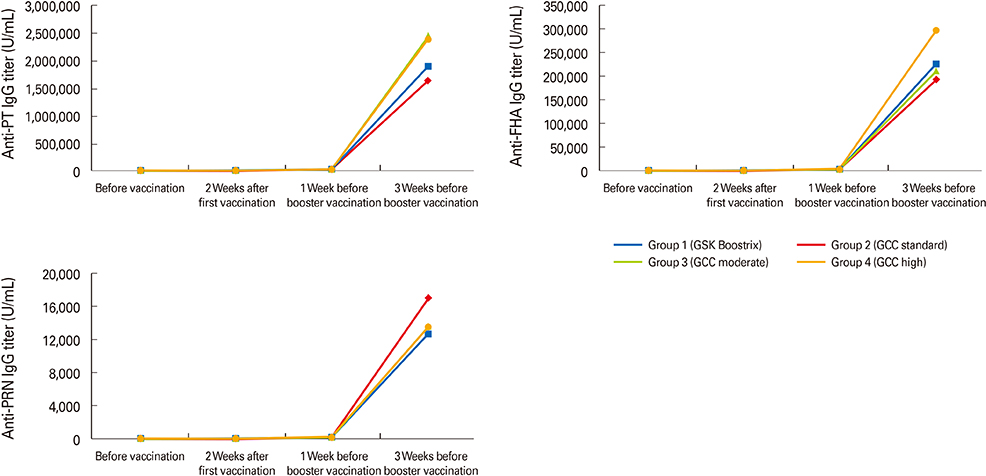
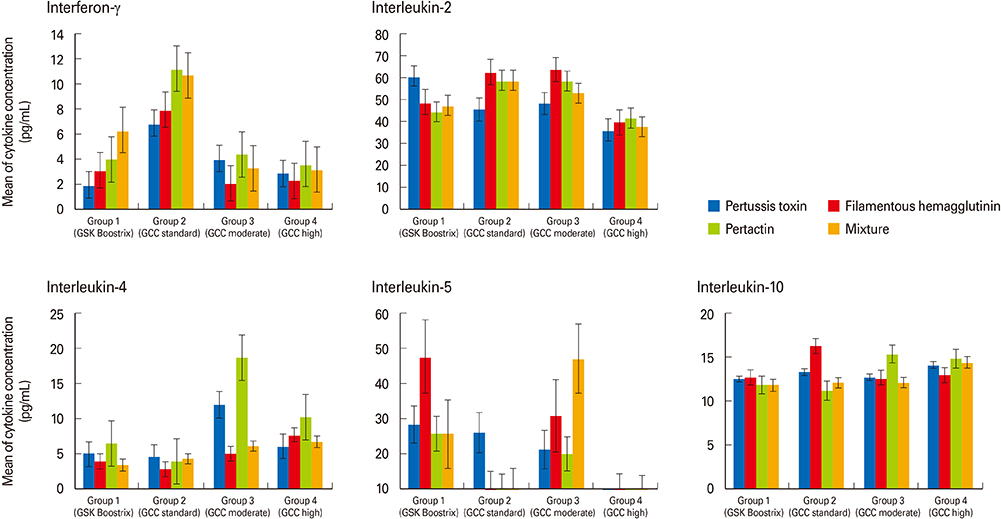
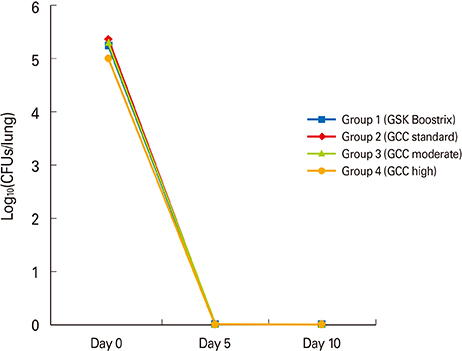
Every group showed significant increases in antibody titers against pertussis antigens such as pertussis toxin, filamentous hemagglutinin, and pertactin after booster vaccination. Spleen cells showed both Th1 and Th2 cell-mediated immune responses stimulated by pertussis antigens in all groups without any significant difference. In the intranasal B. pertussis infection model, bacteria were eradicated in all groups five days after challenge infection.
CONCLUSION
This preliminary study did not show significantly different immunogenicity or protection efficacy of the new GCC Tdap vaccines compared to the commercially available Tdap vaccine, although a more extensive study is necessary to assess the differing efficacies of the new GCC Tdap vaccines.
Keyword
MeSH Terms
Figure
Reference
-
1. Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005; 18:326–382.
Article2. de Melker HE, Schellekens JF, Neppelenbroek SE, Mooi FR, Rümke HC, Conyn-van Spaendonck MA. Reemergence of pertussis in the highly vaccinated population of the Netherlands: observations on surveillance data. Emerg Infect Dis. 2000; 6:348–357.
Article3. Crowcroft NS, Britto J. Whooping cough: a continuing problem. BMJ. 2002; 324:1537–1538.4. Greenberg DP. Pertussis in adolescents: increasing incidence brings attention to the need for booster immunization of adolescents. Pediatr Infect Dis J. 2005; 24:721–728.5. Packard ER, Parton R, Coote JG, Fry NK. Sequence variation and conservation in virulence-related genes of Bordetella pertussis isolates from the UK. J Med Microbiol. 2004; 53(Pt 5):355–365.
Article6. Centers for Disease Control and Prevention (CDC). Updated recommendations for the use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011; 60:13–15.7. Wei SC, Tatti K, Cushing K, et al. Effectiveness of adolescent and adult tetanus, reduced-dose diphtheria, and acellular pertussis vaccine against pertussis. Clin Infect Dis. 2010; 51:315–321.
Article8. Korea Centers for Disease Control and Prevention. Increasing incidence of pertussis in Korea, 2009. Public Health Wkly Rep. 2009; 2:709.9. Report of Yeongam pertussis epidemiological investigation in Korea. Public Health Wkly Rep. 2012; 5:510–512.10. Mills KH, Ryan M, Ryan E, Mahon BP. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect Immun. 1998; 66:594–602.
Article11. Guiso N, Capiau C, Carletti G, Poolman J, Hauser P. Intranasal murine model of Bordetella pertussis infection. I. Prediction of protection in human infants by acellular vaccines. Vaccine. 1999; 17:2366–2376.
Article12. Mills KH. Immunity to Bordetella pertussis. Microbes Infect. 2001; 3:655–677.
Article13. Canthaboo C, Williams L, Xing DK, Corbel MJ. Investigation of cellular and humoral immune responses to whole cell and acellular pertussis vaccines. Vaccine. 2000; 19:637–643.
Article14. Mills KH, Barnard A, Watkins J, Redhead K. Cell-mediated immunity to Bordetella pertussis: role of Th1 cells in bacterial clearance in a murine respiratory infection model. Infect Immun. 1993; 61:399–410.
Article15. WHO Expert Committee on Biological Standardization. WHO technical report series 800. Geneva: World Health Organization;1990.16. European Directorate for the Quality of Medicines. European pharmacopoeia. 4th ed. Strasbourg: Council of Europe;2002.17. Corbel MJ, Xing DK, Kreeftenberg JG. Informal consultation with manufacturers and WHO Ad hoc working group on mouse protection models for acellular pertussis vaccines national institute for biological standards, 12 november 1998. Biologicals. 1999; 27:189–193.
Article18. Corbel MJ, Mastrantonio P, Kreeftenberg JG. Ad hoc Working Group on acellular pertussis vaccines, World Health Organisation, Geneva, 27-28 July 2000. Vaccine. 2001; 20:288–291.
Article19. Corbel MJ, Kreeftenberg JG, Knezevic I. WHO Working Group on the standardisation and control of pertussis vaccines-report of a meeting held on 6-7 May 2003, Ferney Voltaire, France. Vaccine. 2004; 22:293–300.
Article20. Corbel MJ, Xing DK. Toxicity and potency evaluation of pertussis vaccines. Expert Rev Vaccines. 2004; 3:89–101.
Article21. Xing DK, Das RG, Williams L, Canthaboo C, Tremmil J, Corbel MJ. An aerosol challenge model of Bordetella pertussis infection as a potential bioassay for acellular pertussis vaccines. Vaccine. 1999; 17:565–576.
Article22. Capiau C, Poolman J, Hoet B, Bogaerts H, Andre F. Development and clinical testing of multivalent vaccines based on a diphtheria-tetanus-acellular pertussis vaccine: difficulties encountered and lessons learned. Vaccine. 2003; 21:2273–2287.
Article23. Zepp F, Knuf M, Habermehl P, et al. Pertussis-specific cell-mediated immunity in infants after vaccination with a tricomponent acellular pertussis vaccine. Infect Immun. 1996; 64:4078–4084.
Article24. Esposito S, Agliardi T, Giammanco A, et al. Long-term pertussis-specific immunity after primary vaccination with a combined diphtheria, tetanus, tricomponent acellular pertussis, and hepatitis B vaccine in comparison with that after natural infection. Infect Immun. 2001; 69:4516–4520.
Article25. Southern J, Andrews N, Burrage M, Miller E. Immunogenicity and reactogenicity of combined acellular pertussis/tetanus/low dose diphtheria vaccines given as a booster to UK teenagers. Vaccine. 2005; 23:3829–3835.
Article26. Ausiello CM, Urbani F, la Sala A, Lande R, Cassone A. Vaccine and antigen-dependent type 1 and type 2 cytokine induction after primary vaccination of infants with whole-cell or acellular pertussis vaccines. Infect Immun. 1997; 65:2168–2174.
Article27. Redhead K, Watkins J, Barnard A, Mills KH. Effective immunization against Bordetella pertussis respiratory infection in mice is dependent on induction of cell-mediated immunity. Infect Immun. 1993; 61:3190–3198.
Article28. He Q, Tran Minh NN, Edelman K, Viljanen MK, Arvilommi H, Mertsola J. Cytokine mRNA expression and proliferative responses induced by pertussis toxin, filamentous haemagglutinin, and pertactin of Bordetella pertussis in the peripheral blood mononuclear cells of infected and immunized schoolchildren and adults. Infect Immun. 1998; 66:3796–3801.
Article29. Ryan M, Murphy G, Ryan E, et al. Distinct T-cell subtypes induced with whole cell and acellular pertussis vaccines in children. Immunology. 1998; 93:1–10.
Article30. Ryan EJ, Nilsson L, Kjellman N, Gothefors L, Mills KH. Booster immunization of children with an acellular pertussis vaccine enhances Th2 cytokine production and serum IgE responses against pertussis toxin but not against common allergens. Clin Exp Immunol. 2000; 121:193–200.
Article31. Reynolds E, Walker B, Xing D, et al. Laboratory investigation of immune responses to acellular pertussis vaccines when used for boosting adolescents after primary immunisation with whole cell pertussis vaccines: a comparison with data from clinical study. Vaccine. 2006; 24:3248–3257.
Article32. Dolby JM, Thow DC, Standfast AF. The intranasal infection of mice with Bordetella pertussis. J Hyg. 1961; 59:191–204.
Article33. Denoel P, Godfroid F, Guiso N, Hallander H, Poolman J. Comparison of acellular pertussis vaccines-induced immunity against infection due to Bordetella pertussis variant isolates in a mouse model. Vaccine. 2005; 23:5333–5341.
Article34. Bottero D, Gaillard ME, Fingermann M, et al. Pulsed-field gel electrophoresis, pertactin, pertussis toxin S1 subunit polymorphisms, and surfaceome analysis of vaccine and clinical Bordetella pertussis strains. Clin Vaccine Immunol. 2007; 14:1490–1498.
Article35. Komatsu E, Yamaguchi F, Abe A, Weiss AA, Watanabe M. Synergic effect of genotype changes in pertussis toxin and pertactin on adaptation to an acellular pertussis vaccine in the murine intranasal challenge model. Clin Vaccine Immunol. 2010; 17:807–812.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Bordetella pertussis proteoliposome induces protection in mice without affecting the immunogenicity of diphtheria and tetanus toxoids in a trivalent formulation
- Recommendation for the use of newly introduced Tdap vaccine in Korea
- Enzyme-linked immunosorbent assay for detecting anti-pertussis toxin antibody in mouse
- Update on pertussis and pertussis immunization
- Investigation on the Immunity to Pertussis in the Korea