Clin Exp Vaccine Res.
2015 Jan;4(1):68-74. 10.7774/cevr.2015.4.1.68.
Cellular immune response following pre-exposure and postexposure rabies vaccination by intradermal and intramuscular routes
- Affiliations
-
- 1Deptartment of Neurovirology, National Institute of Mental Health and Neurosciences, Bangalore, India. mshampur@hotmail.com
- KMID: 2049109
- DOI: http://doi.org/10.7774/cevr.2015.4.1.68
Abstract
- PURPOSE
Immunization against rabies in humans induces protective neutralizing antibodies; however, the induction of type 1 or type 2 cytokine mediated cellular immune responses following rabies vaccination is not understood. Hence, the present study investigated cellular cytokine responses in vaccinated individuals.
MATERIALS AND METHODS
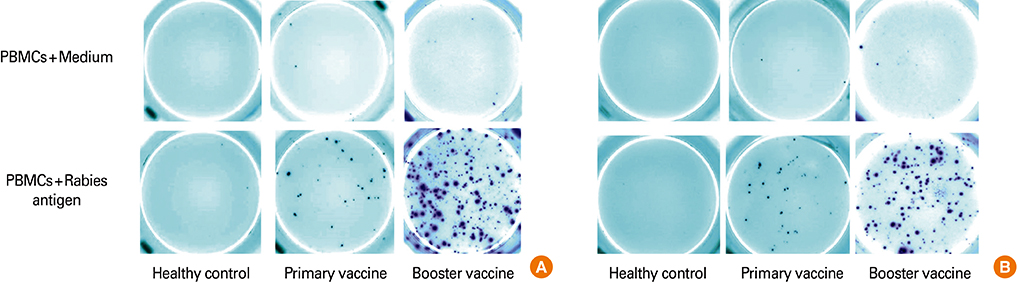
The study groups included healthy rabies antigen naive controls (n=10), individuals who received intradermal primary (n=10) or booster pre-exposure vaccination (n=20) and subjects who received postexposure rabies vaccination either by intradermal (n=18) or intramuscular (n=20) routes. The antigen specific cellular responses were analyzed by stimulating peripheral blood mononuclear cells with a rabies vaccine antigen in the interferon-gamma (IFN-gamma) and interleukin-4 (IL-4) enzyme-linked immunospot (ELISpot) assay. These responses were compared to the rabies virus neutralizing antibody (RVNA) titers that were measured by rapid fluorescent focus inhibition test.
RESULTS
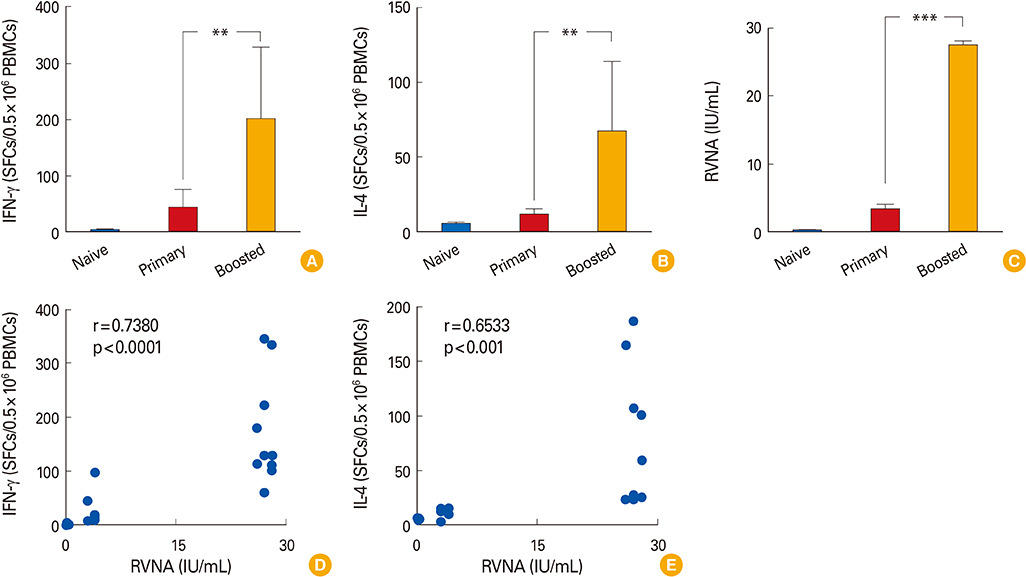
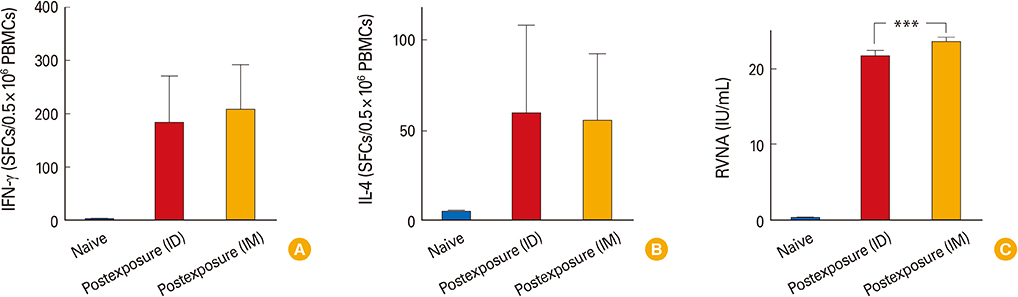
We observed that cellular and humoral immune responses to primary intradermal rabies vaccination could be greatly enhanced by a booster vaccine; and both type 1 and type 2 cytokine responses were significantly elevated. The magnitude of type 1 and type 2 cytokine responses did not differ significantly among the intramuscular and intradermal routes of postexposure vaccination. The number of cells producing IFN-gamma and IL-4 correlated significantly with the levels of RVNA.
CONCLUSION
Both type 1 and type 2 cellular cytokine responses are strongly induced after rabies vaccination and directly correlate with levels of RVNA titers. The neutralizing antibody as well as the type 1 and type 2 cytokine responses may be important for vaccine induced protective responses against rabies.
Keyword
MeSH Terms
Figure
Reference
-
1. World Health Organization. WHO technical report series, No. 982. WHO expert consultation on rabies. Second report. Geneva: World Health Organization;2013.2. Briggs DJ, Mahendra BJ. Public health management of humans at risk. In : Jackson AC, Wunner WH, editors. Rabies. 2nd ed. Amsterdam: Elsevier Academic Press;2007. p. 545–566.3. Madhusudana SN. Rabies. Gurgaon: Macmillan Medical Communications;2011. p. 148–158.4. Ravish HS, Sudarshan MK, Madhusudana SN, et al. Assessing safety and immunogenicity of post-exposure prophylaxis following interchangeability of rabies vaccines in humans. Hum Vaccin Immunother. 2014; 10:1354–1358.
Article5. Flamand A, Raux H, Gaudin Y, Ruigrok RW. Mechanisms of rabies virus neutralization. Virology. 1993; 194:302–313.
Article6. Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 1998; 72:3711–3719.
Article7. Johnson N, Cunningham AF, Fooks AR. The immune response to rabies virus infection and vaccination. Vaccine. 2010; 28:3896–3901.
Article8. Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4(+) T cells in immunity to viruses. Nat Rev Immunol. 2012; 12:136–148.
Article9. Sant AJ, McMichael A. Revealing the role of CD4(+) T cells in viral immunity. J Exp Med. 2012; 209:1391–1395.
Article10. Nair S, Bayer W, Ploquin MJ, Kassiotis G, Hasenkrug KJ, Dittmer U. Distinct roles of CD4+ T cell subpopulations in retroviral immunity: lessons from the Friend virus mouse model. Retrovirology. 2011; 8:76.11. Gans HA, Yasukawa LL, Sung P, et al. Measles humoral and cell-mediated immunity in children aged 5-10 years after primary measles immunization administered at 6 or 9 months of age. J Infect Dis. 2013; 207:574–582.
Article12. Frazer IH, Jones B, Dimitrakakis M, Mackay IR. Intramuscular versus low-dose intradermal hepatitis B vaccine: assessment by humoral and cellular immune response to hepatitis B surface antigen. Med J Aust. 1987; 146:242–245.
Article13. Ghendon Y. The immune response to influenza vaccines. Acta Virol. 1990; 34:295–304.14. Brown D, Fooks AR, Schweiger M. Using intradermal rabies vaccine to boost immunity in people with low rabies antibody levels. Adv Prev Med. 2011; 2011:601789.
Article15. Madhusudana SN, Mani RS. Intradermal vaccination for rabies prophylaxis: conceptualization, evolution, present status and future. Expert Rev Vaccines. 2014; 13:641–655.
Article16. Smith JS, Yager PA, Baer GM. A rapid fluorescent focus inhibition test (RFFIT) for determining rabies virus-neutralising antibody. In : Meslin FX, Kaplan MM, Koprowski H, editors. Laboratory techniques in rabies. 4th ed. Geneva: World Health Organization;1996. p. 181–191.17. Thraenhart O, Kreuzfelder E, Hillebrandt M, et al. Long-term humoral and cellular immunity after vaccination with cell culture rabies vaccines in man. Clin Immunol Immunopathol. 1994; 71:287–292.
Article18. Moore SM, Hanlon CA. Rabies-specific antibodies: measuring surrogates of protection against a fatal disease. PLoS Negl Trop Dis. 2010; 4:e595.
Article19. Hooper DC, Roy A, Kean RB, Phares TW, Barkhouse DA. Therapeutic immune clearance of rabies virus from the CNS. Future Virol. 2011; 6:387–397.
Article20. Neves PC, Santos JR, Tubarao LN, Bonaldo MC, Galler R. Early IFN-gamma production after YF 17D vaccine virus immunization in mice and its association with adaptive immune responses. PLoS One. 2013; 8:e81953.
Article21. Calarota SA, Baldanti F. Enumeration and characterization of human memory T cells by enzyme-linked immunospot assays. Clin Dev Immunol. 2013; 2013:637649.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Five Year Experience of Preexposure and Postexposure Rabies Prophylaxis in Korean Children at the National Medical Center
- General Features and Post-Exposure Prophylaxis of Rabies
- Immunogenicity and efficacy of a plasmid DNA rabies vaccine incorporating Myd88 as a genetic adjuvant
- Epidemiology and Prevention Strategies of Rabies in Korea
- Long-term immunogenicity of the influenza vaccine at reduced intradermal and full intramuscular doses among healthy young adults