Clin Exp Vaccine Res.
2015 Jan;4(1):23-45. 10.7774/cevr.2015.4.1.23.
Polyionic vaccine adjuvants: another look at aluminum salts and polyelectrolytes
- Affiliations
-
- 1PharmAthene, Inc., Annapolis, MD, USA. bradford.powell@pharmathene.com
- 2Institute for Bioscience and Biotechnology Research, University of Maryland, College Park, MD, USA.
- KMID: 2049105
- DOI: http://doi.org/10.7774/cevr.2015.4.1.23
Abstract
- Adjuvants improve the adaptive immune response to a vaccine antigen by modulating innate immunity or facilitating transport and presentation. The selection of an appropriate adjuvant has become vital as new vaccines trend toward narrower composition, expanded application, and improved safety. Functionally, adjuvants act directly or indirectly on antigen presenting cells (APCs) including dendritic cells (DCs) and are perceived as having molecular patterns associated either with pathogen invasion or endogenous cell damage (known as pathogen associated molecular patterns [PAMPs] and damage associated molecular patterns [DAMPs]), thereby initiating sensing and response pathways. PAMP-type adjuvants are ligands for toll-like receptors (TLRs) and can directly affect DCs to alter the strength, potency, speed, duration, bias, breadth, and scope of adaptive immunity. DAMP-type adjuvants signal via proinflammatory pathways and promote immune cell infiltration, antigen presentation, and effector cell maturation. This class of adjuvants includes mineral salts, oil emulsions, nanoparticles, and polyelectrolytes and comprises colloids and molecular assemblies exhibiting complex, heterogeneous structures. Today innovation in adjuvant technology is driven by rapidly expanding knowledge in immunology, cross-fertilization from other areas including systems biology and materials sciences, and regulatory requirements for quality, safety, efficacy and understanding as part of the vaccine product. Standardizations will aid efforts to better define and compare the structure, function and safety of adjuvants. This article briefly surveys the genesis of adjuvant technology and then re-examines polyionic macromolecules and polyelectrolyte materials, adjuvants currently not known to employ TLR. Specific updates are provided for aluminum-based formulations and polyelectrolytes as examples of improvements to the oldest and emerging classes of vaccine adjuvants in use.
Keyword
MeSH Terms
-
Adaptive Immunity
Adjuvants, Immunologic
Allergy and Immunology
Aluminum Hydroxide
Aluminum*
Antigen Presentation
Antigen-Presenting Cells
Bias (Epidemiology)
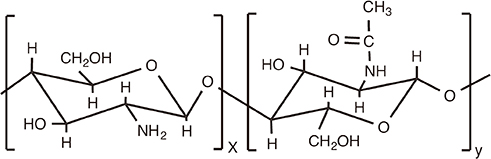
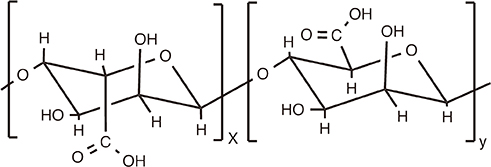
Chitosan
Colloids
Dendritic Cells
Emulsions
Immunity, Innate
Ligands
Nanoparticles
Polymers
Receptors, Pattern Recognition
Salts*
Systems Biology
Toll-Like Receptors
Vaccines
Adjuvants, Immunologic
Aluminum
Aluminum Hydroxide
Chitosan
Colloids
Emulsions
Ligands
Polymers
Receptors, Pattern Recognition
Salts
Toll-Like Receptors
Vaccines
Figure
Reference
-
1. van Panhuis WG, Grefenstette J, Jung SY, et al. Contagious diseases in the United States from 1888 to the present. N Engl J Med. 2013; 369:2152–2158.
Article2. Rappuoli R, Pizza M, Del Giudice G, De Gregorio E. Vaccines, new opportunities for a new society. Proc Natl Acad Sci U S A. 2014; 111:12288–12293.
Article3. World Health Organization. Global Vaccine Action Plan 2011-2020. Geneva: WHO;2013.4. Black RE, Cousens S, Johnson HL, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010; 375:1969–1987.
Article5. Rusnak JM, Kortepeter MG, Hawley RJ, Anderson AO, Boudreau E, Eitzen E. Risk of occupationally acquired illnesses from biological threat agents in unvaccinated laboratory workers. Biosecur Bioterror. 2004; 2:281–293.
Article6. World Health Organization. Global vaccine action plan: monitoring, evaluation and accountability annual report. Geneva: WHO;2014.7. Mahadevan M, Navarro-Locsin G, Tan HK, et al. A review of the burden of disease due to otitis media in the Asia-Pacific. Int J Pediatr Otorhinolaryngol. 2012; 76:623–635.
Article8. Memish ZA. Meningococcal disease and travel. Clin Infect Dis. 2002; 34:84–90.
Article9. Hinman A. Eradication of vaccine-preventable diseases. Annu Rev Public Health. 1999; 20:211–229.
Article10. Hennessey K, Schluter WW, Wang X, et al. Are we there yet? Assessing achievement of vaccine-preventable disease goals in WHO's Western Pacific Region. Vaccine. 2014; 32:4259–4266.
Article11. Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc (Bayl Univ Med Cent). 2005; 18:21–25.
Article12. Henderson DA. The eradication of smallpox: an overview of the past, present, and future. Vaccine. 2011; 29:Suppl 4. D7–D9.13. Anand A, Pallansch MA, Estivariz CF, Gary H, Wassilak SG. Estimating the likely coverage of inactivated poliovirus vaccine in routine immunization: evidence from demographic and health surveys. J Infect Dis. 2014; 210:Suppl 1. S465–S474.
Article14. Klepac P, Metcalf CJ, McLean AR, Hampson K. Towards the endgame and beyond: complexities and challenges for the elimination of infectious diseases. Philos Trans R Soc Lond B Biol Sci. 2013; 368:20120137.
Article15. Lievano F, Galea SA, Thornton M, et al. Measles, mumps, and rubella virus vaccine (M-M-RII): a review of 32 years of clinical and postmarketing experience. Vaccine. 2012; 30:6918–6926.
Article16. Alicino C, Merlano C, Zappettini S, et al. Routine surveillance of adverse events following immunization as an important tool to monitor vaccine safety: the two-years' experience of the Liguria Region, Italy. Hum Vaccin Immunother. 2015; 11:91–94.
Article17. Arguedas A, Soley C, Abdelnour A, et al. Assessment of the safety, tolerability and kinetics of the immune response to A/H1N1v vaccine formulations with and without adjuvant in healthy pediatric subjects from 3 through 17 years of age. Hum Vaccin. 2011; 7:58–66.
Article18. Chang S, O'Connor PM, Slade BA, Woo EJ. U.S. Postlicensure safety surveillance for adolescent and adult tetanus, diphtheria and acellular pertussis vaccines: 2005-2007. Vaccine. 2013; 31:1447–1452.
Article19. Cottin P, Niedrig M, Domingo C. Safety profile of the yellow fever vaccine Stamaril(R): a 17-year review. Expert Rev Vaccines. 2013; 12:1351–1368.20. DeStefano F. Vaccine Safety Datalink Research Group. The Vaccine Safety Datalink project. Pharmacoepidemiol Drug Saf. 2001; 10:403–406.
Article21. Lee CJ, Lee LH, Lu CH, Huang YJ, Chu ML. Safety monitoring in vaccine development and immunization. Acta Paediatr Taiwan. 2006; 47:7–13.22. Macartney KK, Chiu C, Georgousakis M, Brotherton JM. Safety of human papillomavirus vaccines: a review. Drug Saf. 2013; 36:393–412.
Article23. Tseng HF, Liu A, Sy L, et al. Safety of zoster vaccine in adults from a large managed-care cohort: a Vaccine Safety Datalink study. J Intern Med. 2012; 271:510–520.
Article24. Nguyen TH, Vu MH, Nguyen VC, et al. A reduction in chronic hepatitis B virus infection prevalence among children in Vietnam demonstrates the importance of vaccination. Vaccine. 2014; 32:217–222.
Article25. Teleb N, Lebo E, Ahmed H, et al. Progress toward measles elimination: Eastern Mediterranean Region, 2008-2012. MMWR Morb Mortal Wkly Rep. 2014; 63:511–515.26. Kapoor R, Kottilil S. Strategies to eliminate HBV infection. Future Virol. 2014; 9:565–585.
Article27. Andre FE, Booy R, Bock HL, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008; 86:140–146.
Article28. Garcon N, Hem S, Friede M. Evolution of adjuvants across the centuries. In : Plotkin S, Ornstein W, Offit P, editors. Vaccines. 6th ed. Philadelphia: Saunders Elsevier;2013. p. 58–70.29. Glenny AT, Sudmersen HJ. Notes on the production of immunity to diphtheria toxin. J Hyg (Lond). 1921; 20:176–220.
Article30. Emil von Behring: the founder of serum therapy [Internet]. Nobel Foundation;2001. cited 2014 Nov 18. Available from: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1901/behring-article.html.31. Kantha SS. The legacy of von Behring and Kitasato. Immunol Today. 1992; 13:374.
Article32. Plotkin SL, Plotkin SA. A short history of vaccination. In : Plotkin S, Ornstein W, Offit P, editors. Vaccines. 6th ed. Philadelphia: Saunders Elsevier;2013. p. 1–13.33. The tetanus cases in Camden, N.J. JAMA. 1901; 37:1539–1540.34. U.S. Food and Drug Administration. Biologics centennial: 100 years of biologics regulation [Internet]. Silver Spring: FDA;2002. cited 2014 Nov 18. Available from: http://www.fda.gov/AboutFDA/WhatWeDo/History/ProductRegulation/SelectionsFromFDLIUpdateSeriesonFDAHistory/ucm091754.htm.35. Dudzinski DM. Reflections on historical, scientific, and legal issues relevant to designing approval pathways for generic versions of recombinant protein-based therapeutics and monoclonal antibodies. Food Drug Law J. 2005; 60:143–260.36. Ramon G. Regarding the flocculant ability and immunizing properties of a diphtheria toxin rendered atoxic. C R Hebd Seances Acad Sci. 1923; 177:1338–1340.37. Glenny AT, Pope CG, Waddington H, Wallace U. Immunological notes: XVII-XXIV. J Pathol Bacteriol. 1926; 29:31–40.
Article38. Park WH, Schroder MC. Diphtheria toxin-antitoxin and toxoid: a comparison. Am J Public Health Nations Health. 1932; 22:7–16.39. Baker JN, Gill DG. Precipitated toxoid as an immunizing agent against diphtheria. Am J Public Health Nations Health. 1934; 24:22–24.
Article40. Ericsson H. Purification and adsorption of diphtheria toxoid. Nature. 1946; 158:350.
Article41. Holt LB. Purified precipitated diphtheria toxoid of constant composition. Lancet. 1947; 1:282–285.42. Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011; 11:865–872.
Article43. Freund J. The effect of paraffin oil and mycobacteria on antibody formation and sensitization: a review. Am J Clin Pathol. 1951; 21:645–656.
Article44. Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004; 82:488–496.
Article45. Stuart-Harris CH. Adjuvant influenza vaccines. Bull World Health Organ. 1969; 41:617–621.46. O'Hagan DT, Ott GS, Nest GV, Rappuoli R, Giudice GD. The history of MF59((R)) adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines. 2013; 12:13–30.47. Morel S, Didierlaurent A, Bourguignon P, et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011; 29:2461–2473.
Article48. Caillet C, Piras F, Bernard MC, et al. AF03-adjuvanted and non-adjuvanted pandemic influenza A (H1N1) 2009 vaccines induce strong antibody responses in seasonal influenza vaccine-primed and unprimed mice. Vaccine. 2010; 28:3076–3079.
Article49. Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine. 2011; 29:9563–9572.
Article50. Rietschel ET, Cavaillon JM. Richard Pfeiffer and Alexandre Besredka: creators of the concept of endotoxin and anti-endotoxin. Microbes Infect. 2003; 5:1407–1414.
Article51. Nauts HC, Swift WE, Coley BL. The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, M.D., reviewed in the light of modern research. Cancer Res. 1946; 6:205–216.52. Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001; 1:135–145.
Article53. Medzhitov R, Janeway CA Jr. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997; 9:4–9.
Article54. Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013; 19:1597–1608.
Article55. De Gregorio E, Rappuoli R. From empiricism to rational design: a personal perspective of the evolution of vaccine development. Nat Rev Immunol. 2014; 14:505–514.
Article56. Petrovsky N. Vaccine adjuvants: in search of new paradigms. Interview by Jenaid Rees. Expert Rev Vaccines. 2013; 12:723–726.57. Koff WC, Burton DR, Johnson PR, et al. Accelerating next-generation vaccine development for global disease prevention. Science. 2013; 340:1232910.
Article58. Brito LA, Malyala P, O'Hagan DT. Vaccine adjuvant formulations: a pharmaceutical perspective. Semin Immunol. 2013; 25:130–145.
Article59. Li L, Saade F, Petrovsky N. The future of human DNA vaccines. J Biotechnol. 2012; 162:171–182.
Article60. Williams A, Flavell RA, Eisenbarth SC. The role of NOD-like receptors in shaping adaptive immunity. Curr Opin Immunol. 2010; 22:34–40.
Article61. Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010; 33:492–503.
Article62. Carter D, Reed SG. Role of adjuvants in modeling the immune response. Curr Opin HIV AIDS. 2010; 5:409–413.
Article63. Rappuoli R. Reverse vaccinology. Curr Opin Microbiol. 2000; 3:445–450.
Article64. Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000; 176:154–170.
Article65. Wetzler LM. Innate immune function of the neisserial porins and the relationship to vaccine adjuvant activity. Future Microbiol. 2010; 5:749–758.
Article66. Massari P, Gunawardana J, Liu X, Wetzler LM. Meningococcal porin PorB prevents cellular apoptosis in a toll-like receptor 2- and NF-kappaB-independent manner. Infect Immun. 2010; 78:994–1003.
Article67. Taylor DN, Treanor JJ, Sheldon EA, et al. Development of VAX128, a recombinant hemagglutinin (HA) influenza-flagellin fusion vaccine with improved safety and immune response. Vaccine. 2012; 30:5761–5769.
Article68. De Gregorio E, D'Oro U, Wack A. Immunology of TLR-independent vaccine adjuvants. Curr Opin Immunol. 2009; 21:339–345.
Article69. Kay E, Scotland RS, Whiteford JR. Toll-like receptors: Role in inflammation and therapeutic potential. Biofactors. 2014; 40:284–294.
Article70. Cervantes JL, Weinerman B, Basole C, Salazar JC. TLR8: the forgotten relative revindicated. Cell Mol Immunol. 2012; 9:434–438.
Article71. Carta S, Tassi S, Pettinati I, Delfino L, Dinarello CA, Rubartelli A. The rate of interleukin-1beta secretion in different myeloid cells varies with the extent of redox response to Toll-like receptor triggering. J Biol Chem. 2011; 286:27069–27080.
Article72. Isakov E, Weisman-Shomer P, Benhar M. Suppression of the pro-inflammatory NLRP3/interleukin-1beta pathway in macrophages by the thioredoxin reductase inhibitor auranofin. Biochim Biophys Acta. 2014; 1840:3153–3161.
Article73. Dasu MR, Isseroff RR. Toll-like receptors in wound healing: location, accessibility, and timing. J Invest Dermatol. 2012; 132:1955–1958.
Article74. Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010; 10:826–837.
Article75. Muralidharan S, Mandrekar P. Cellular stress response and innate immune signaling: integrating pathways in host defense and inflammation. J Leukoc Biol. 2013; 94:1167–1184.
Article76. Mount A, Koernig S, Silva A, Drane D, Maraskovsky E, Morelli AB. Combination of adjuvants: the future of vaccine design. Expert Rev Vaccines. 2013; 12:733–746.
Article77. Kuroda E, Coban C, Ishii KJ. Particulate adjuvant and innate immunity: past achievements, present findings, and future prospects. Int Rev Immunol. 2013; 32:209–220.
Article78. Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011; 469:221–225.
Article79. Flach TL, Ng G, Hari A, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 2011; 17:479–487.
Article80. Seubert A, Calabro S, Santini L, et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci U S A. 2011; 108:11169–11174.
Article81. Wilson NS, Duewell P, Yang B, et al. Inflammasome-dependent and -independent IL-18 production mediates immunity to the ISCOMATRIX adjuvant. J Immunol. 2014; 192:3259–3268.
Article82. Baroja-Mazo A, Martin-Sanchez F, Gomez AI, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014; 15:738–748.
Article83. McKee AS, Munks MW, MacLeod MK, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009; 183:4403–4414.
Article84. Hutchison S, Benson RA, Gibson VB, Pollock AH, Garside P, Brewer JM. Antigen depot is not required for alum adjuvanticity. FASEB J. 2012; 26:1272–1279.
Article85. Watkinson A, Soliakov A, Ganesan A, et al. Increasing the potency of an alhydrogel-formulated anthrax vaccine by minimizing antigen-adjuvant interactions. Clin Vaccine Immunol. 2013; 20:1659–1668.
Article86. Iyer V, Hu L, Liyanage MR, et al. Preformulation characterization of an aluminum salt-adjuvanted trivalent recombinant protein-based vaccine candidate against Streptococcus pneumoniae. J Pharm Sci. 2012; 101:3078–3090.
Article87. Soliakov A, Kelly IF, Lakey JH, Watkinson A. Anthrax sub-unit vaccine: the structural consequences of binding rPA83 to Alhydrogel(R). Eur J Pharm Biopharm. 2012; 80:25–32.
Article88. Exley C, Siesjo P, Eriksson H. The immunobiology of aluminium adjuvants: how do they really work. Trends Immunol. 2010; 31:103–109.
Article89. Lu F, Hogenesch H. Kinetics of the inflammatory response following intramuscular injection of aluminum adjuvant. Vaccine. 2013; 31:3979–3986.
Article90. Rimaniol AC, Gras G, Verdier F, et al. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine. 2004; 22:3127–3135.
Article91. Mold M, Eriksson H, Siesjo P, Darabi A, Shardlow E, Exley C. Unequivocal identification of intracellular aluminium adjuvant in a monocytic THP-1 cell line. Sci Rep. 2014; 4:6287.
Article92. He Y. Vaccine adjuvant informatics: from data integration and analysis to rational vaccine adjuvant design. Front Immunol. 2014; 5:32.
Article93. Sayers S, Ulysse G, Xiang Z, He Y. Vaxjo: a web-based vaccine adjuvant database and its application for analysis of vaccine adjuvants and their uses in vaccine development. J Biomed Biotechnol. 2012; 2012:831486.
Article94. Hartung DM, Zarin DA, Guise JM, McDonagh M, Paynter R, Helfand M. Reporting discrepancies between the ClinicalTrials.gov results database and peer-reviewed publications. Ann Intern Med. 2014; 160:477–483.
Article95. Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database--update and key issues. N Engl J Med. 2011; 364:852–860.
Article96. Levast B, Berri M, Wilson HL, Meurens F, Salmon H. Development of gut immunoglobulin A production in piglet in response to innate and environmental factors. Dev Comp Immunol. 2014; 44:235–244.
Article97. Gupta RK, Rost BE, Relyveld E, Siber GR. Adjuvant properties of aluminum and calcium compounds. Pharm Biotechnol. 1995; 6:229–248.
Article98. Hem SL, White JL. Structure and properties of aluminum-containing adjuvants. Pharm Biotechnol. 1995; 6:249–276.
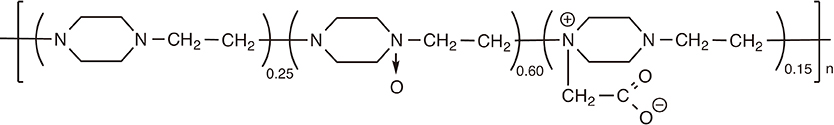
Article99. Li X, Aldayel AM, Cui Z. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J Control Release. 2014; 173:148–157.
Article100. Barbaud A, Deschildre A, Waton J, Raison-Peyron N, Trechot P. Hypersensitivity and vaccines: an update. Eur J Dermatol. 2013; 23:135–141.
Article101. Pittman PR, Kim-Ahn G, Pifat DY, et al. Anthrax vaccine: immunogenicity and safety of a dose-reduction, route-change comparison study in humans. Vaccine. 2002; 20:1412–1420.
Article102. Hogenesch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012; 3:406.
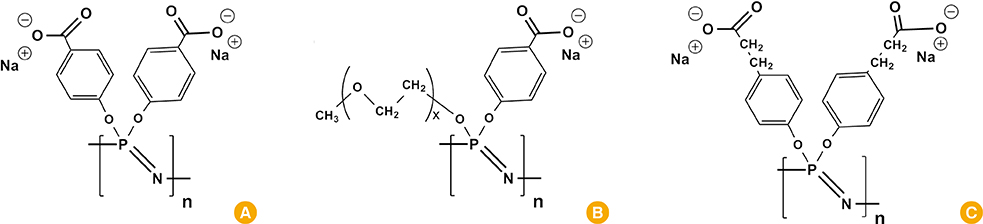
Article103. Jiang D, Premachandra GS, Johnston C, Hem SL. Structure and adsorption properties of commercial calcium phosphate adjuvant. Vaccine. 2004; 23:693–698.
Article104. Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008; 453:1122–1126.
Article105. Sharp FA, Ruane D, Claass B, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A. 2009; 106:870–875.
Article106. Schinwald A, Donaldson K. Use of back-scatter electron signals to visualise cell/nanowires interactions in vitro and in vivo: frustrated phagocytosis of long fibres in macrophages and compartmentalisation in mesothelial cells in vivo. Part Fibre Toxicol. 2012; 9:34.
Article107. Williams GR, Fierens K, Preston SG, et al. Immunity induced by a broad class of inorganic crystalline materials is directly controlled by their chemistry. J Exp Med. 2014; 211:1019–1025.
Article108. Sun B, Ji Z, Liao YP, et al. Engineering an effective immune adjuvant by designed control of shape and crystallinity of aluminum oxyhydroxide nanoparticles. ACS Nano. 2013; 7:10834–10849.
Article109. Mori A, Oleszycka E, Sharp FA, et al. The vaccine adjuvant alum inhibits IL-12 by promoting PI3 kinase signaling while chitosan does not inhibit IL-12 and enhances Th1 and Th17 responses. Eur J Immunol. 2012; 42:2709–2719.
Article110. Neumann S, Burkert K, Kemp R, Rades T, Rod Dunbar P, Hook S. Activation of the NLRP3 inflammasome is not a feature of all particulate vaccine adjuvants. Immunol Cell Biol. 2014; 92:535–542.
Article111. Lu F, Boutselis I, Borch RF, Hogenesch H. Control of antigen-binding to aluminum adjuvants and the immune response with a novel phosphonate linker. Vaccine. 2013; 31:4362–4367.
Article112. Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004; 82:497–505.
Article113. Kabanov VA. From synthetic polyelectrolytes to polymer-subunit vaccines. Pure Appl Chem. 2004; 76:1659–1677.
Article114. Andrianov AK. Polyphosphazene vaccine delivery vehicles: state of development and perspectives. In : Andrianov AK, editor. Polyphosphazenes for biomedical applications. Hoboken: John Wiley & Sons Inc.;2009. p. 47–63.115. Muzzarelli RA. Chitins and chitosans as immunoadjuvants and non-allergenic drug carriers. Mar Drugs. 2010; 8:292–312.
Article116. Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010; 62:59–82.
Article117. Jayakumar R, Prabaharan M, Sudheesh Kumar PT, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv. 2011; 29:322–337.
Article118. You J, Li W, Yu C, et al. Amphiphilically modified chitosan cationic nanoparticles for drug delivery. J Nanopart Res. 2013; 15:2123.
Article119. Nishimura K, Nishimura S, Nishi N, Saiki I, Tokura S, Azuma I. Immunological activity of chitin and its derivatives. Vaccine. 1984; 2:93–99.
Article120. Lee CG. Chitin, chitinases and chitinase-like proteins in allergic inflammation and tissue remodeling. Yonsei Med J. 2009; 50:22–30.
Article121. Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011; 73:479–501.
Article122. Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007; 25:2085–2094.
Article123. Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances the immunoadjuvant properties of GM-CSF. Vaccine. 2007; 25:8673–8686.
Article124. Scherliess R, Buske S, Young K, Weber B, Rades T, Hook S. In vivo evaluation of chitosan as an adjuvant in subcutaneous vaccine formulations. Vaccine. 2013; 31:4812–4819.
Article125. Vasiliev YM. Chitosan-based vaccine adjuvants: incomplete characterization complicates preclinical and clinical evaluation. Expert Rev Vaccines. 2015; 14:37–53.
Article126. Murano E. Use of natural polysaccharides in the microencapsulation techniques. J Appl Ichthyol. 1998; 14:245–249.
Article127. Dobakhti F, Naghibi T, Taghikhani M, et al. Adjuvanticity effect of sodium alginate on subcutaneously injected BCG in BALB/c mice. Microbes Infect. 2009; 11:296–301.
Article128. Mata E, Igartua M, Patarroyo ME, Pedraz JL, Hernandez RM. Enhancing immunogenicity to PLGA microparticulate systems by incorporation of alginate and RGD-modified alginate. Eur J Pharm Sci. 2011; 44:32–40.
Article129. Dyakonova VA, Dambaeva SV, Pinegin BV, Khaitov RM. Study of interaction between the polyoxidonium immunomodulator and the human immune system cells. Int Immunopharmacol. 2004; 4:1615–1623.
Article130. Dambaeva SV, Mazurov DV, Golubeva NM, D'Yakonova VA, Pinegin BV, Khaitov RM. Effect of polyoxidonium on the phagocytic activity of human peripheral blood leukocytes. Russ J Immunol. 2003; 8:53–60.131. Denisov AA, Korobovtseva YS, Karpova OM, et al. Immunopotentiation of live brucellosis vaccine by adjuvants. Vaccine. 2010; 28:Suppl 5. F17–F22.
Article132. Toptygina A, Semikina E, Alioshkin V. Influence of an immunopotentiator polyoxidonium on cytokine profile and antibody production in children vaccinated with Priorix. Arch Physiol Biochem. 2012; 118:197–203.
Article133. Andrianov AK, Marin A, Roberts BE. Polyphosphazene polyelectrolytes: a link between the formation of noncovalent complexes with antigenic proteins and immunostimulating activity. Biomacromolecules. 2005; 6:1375–1379.
Article134. Andrianov AK, Chen J, LeGolvan MP. Poly(dichlorophosphazene) as a precursor for biologically active polyphosphazenes: synthesis, characterization, and stabilization. Macromolecules. 2004; 37:414–420.
Article135. Andrianov AK, LeGolvan MP. Characterization of poly [di(carboxylatophenoxy)-phosphazene] by an aqueous gel permeation chromatography. J Appl Polym Sci. 1996; 60:2289–2295.
Article136. Andrianov AK, Svirkin YY, LeGolvan MP. Synthesis and biologically relevant properties of polyphosphazene polyacids. Biomacromolecules. 2004; 5:1999–2006.
Article137. Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A. Polyphosphazene immunoadjuvants for intradermal vaccine delivery. In : Andrianov AK, editor. Polyphosphazene for biomedical applications. Hoboken: John Wiley & Sons Inc.;2009. p. 101–116.138. Andrianov AK, Decollibus DP, Marin A, Webb A, Griffin Y, Webby RJ. PCPP-formulated H5N1 influenza vaccine displays improved stability and dose-sparing effect in lethal challenge studies. J Pharm Sci. 2011; 100:1436–1443.
Article139. Payne LG, Jenkins SA, Woods AL, et al. Poly[di(carboxylatophenoxy)phosphazene] (PCPP) is a potent immunoadjuvant for an influenza vaccine. Vaccine. 1998; 16:92–98.
Article140. Payne LG, Van Nest G, Barchfeld GL, Siber GR, Gupta RK, Jenkins SA. PCPP as a parenteral adjuvant for diverse antigens. Dev Biol Stand. 1998; 92:79–87.141. Mutwiri G, Benjamin P, Soita H, et al. Poly[di(sodium carboxylatoethylphenoxy)phosphazene] (PCEP) is a potent enhancer of mixed Th1/Th2 immune responses in mice immunized with influenza virus antigens. Vaccine. 2007; 25:1204–1213.
Article142. Lu Y, Salvato MS, Pauza CD, et al. Utility of SHIV for testing HIV-1 vaccine candidates in macaques. J Acquir Immune Defic Syndr Hum Retrovirol. 1996; 12:99–106.
Article143. Wu JY, Wade WF, Taylor RK. Evaluation of cholera vaccines formulated with toxin-coregulated pilin peptide plus polymer adjuvant in mice. Infect Immun. 2001; 69:7695–7702.
Article144. Le Cam NN, Ronco J, Francon A, Blondeau C, Fanget B. Adjuvants for influenza vaccine. Res Immunol. 1998; 149:19–23.
Article145. Plotkin SA. Vaccines: past, present and future. Nat Med. 2005; 11:4 Suppl. S5–11.
Article146. Ison MG, Mills J, Openshaw P, Zambon M, Osterhaus A, Hayden F. Current research on respiratory viral infections: Fourth International Symposium. Antiviral Res. 2002; 55:227–278.
Article147. Kim JH, Kirsch EA, Gilliam B. A phase I, open label, dose ranging trial of the Pasteur Merieux Connaught (PMC) oligomeric HIV-1 Gp160mn/LAI-2 vaccine in HIV seronegative adults. Abstracts of the 37th Annual Meeting of the Infectious Diseases Society of America. Philadelphia, PA, USA: 1999. 11. 18. 21.148. Mutwiri G, Benjamin P, Soita H, Babiuk LA. Co-administration of polyphosphazenes with CpG oligodeoxynucleotides strongly enhances immune responses in mice immunized with hepatitis B virus surface antigen. Vaccine. 2008; 26:2680–2688.
Article149. Istrate C, Hinkula J, Charpilienne A, et al. Parenteral administration of RF 8-2/6/7 rotavirus-like particles in a one-dose regimen induce protective immunity in mice. Vaccine. 2008; 26:4594–4601.
Article150. Palmer CD, Ninkovic J, Prokopowicz ZM, et al. The effect of stable macromolecular complexes of ionic polyphosphazene on HIV Gag antigen and on activation of human dendritic cells and presentation to T-cells. Biomaterials. 2014; 35:8876–8886.
Article151. Andrianov AK, Sargent JR, Sule SS, et al. Synthesis, physico-chemical properties and immunoadjuvant activity of water-soluble phosphazene polyacids. J Bioact Compat Polym. 1998; 13:243–256.
Article152. Andrianov AK, Marin A, Chen J. Synthesis, properties, and biological activity of poly[di(sodium carboxylatoethylphenoxy)phosphazene]. Biomacromolecules. 2006; 7:394–399.
Article153. Johansson E, Istrate C, Charpilienne A, et al. Amount of maternal rotavirus-specific antibodies influence the outcome of rotavirus vaccination of newborn mice with virus-like particles. Vaccine. 2008; 26:778–785.
Article154. Awate S, Wilson HL, Lai K, Babiuk LA, Mutwiri G. Activation of adjuvant core response genes by the novel adjuvant PCEP. Mol Immunol. 2012; 51:292–303.
Article155. Awate S, Wilson HL, Singh B, Babiuk LA, Mutwiri G. The adjuvant PCEP induces recruitment of myeloid and lymphoid cells at the injection site and draining lymph node. Vaccine. 2014; 32:2420–2427.
Article156. Andrianov AK, DeCollibus DP, Gillis HA, et al. Poly[di (carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci U S A. 2009; 106:18936–18941.
Article157. Andrianov AK, Marin A, DeCollibus DP. Microneedles with intrinsic immunoadjuvant properties: microfabrication, protein stability, and modulated release. Pharm Res. 2011; 28:58–65.
Article158. Vaccines Europe. The vaccine industry in figures [Internet]. Brussels: EFPIA/Vaccines Europe. 2014. cited 2014 Nov 18. Available from: http://www.vaccineseurope.eu/about-vaccines-europe/vaccines-europe-in-figures/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vaccine adjuvant materials for cancer immunotherapy and control of infectious disease
- Recent Advances of Vaccine Adjuvants for Infectious Diseases
- Evaluation of precipitation time of the aluminum salts adsorbed potentially frozen vaccines used in the Polish National Immunization Schedule for their pre-qualification before the administration
- A Current Research Insight into Function and Development of Adjuvants
- The development of mucosal vaccines for both mucosal and systemic immune induction and the roles played by adjuvants