Clin Exp Vaccine Res.
2014 Jan;3(1):42-49. 10.7774/cevr.2014.3.1.42.
Microneedle patches for vaccine delivery
- Affiliations
-
- 1Department of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea. dohnanyi@kaist.ac.kr
- KMID: 2049095
- DOI: http://doi.org/10.7774/cevr.2014.3.1.42
Abstract
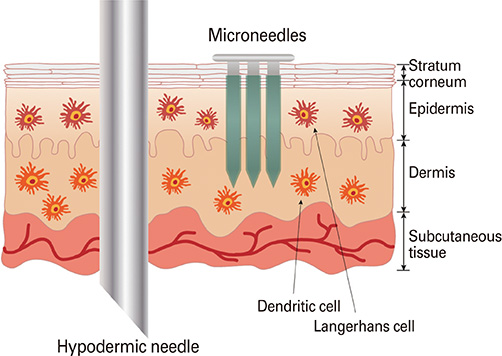
- In today's medical industry, the range of vaccines that exist for administration in humans represents an eclectic variety of forms and immunologic mechanisms. Namely, these are the live attenuated viruses, inactivated viruses, subunit proteins, and virus-like particles for treating virus-caused diseases, as well as the bacterial-based polysaccharide, protein, and conjugated vaccines. Currently, a new approach to vaccination is being investigated with the concept of DNA vaccines. As an alternative delivery route to enhance the vaccination efficacy, microneedles have been devised to target the rich network of immunologic antigen-presenting cells in the dermis and epidermis layers under the skin. Numerous studies have outlined the parameters of microneedle delivery of a wide range of vaccines, revealing comparable or higher immunogenicity to conventional intramuscular routes, overall level of stability, and dose-sparing advantages. Furthermore, recent mechanism studies have begun to successfully elucidate the biological mechanisms behind microneedle vaccination. This paper describes the current status of microneedle vaccine research.
MeSH Terms
Figure
Cited by 1 articles
-
Microneedles: quick and easy delivery methods of vaccines
Ki Mun Kwon, Su-Min Lim, Seulgi Choi, Da-Hee Kim, Hee-Eun Jin, Grace Jee, Kee-Jong Hong, Joo Young Kim
Clin Exp Vaccine Res. 2017;6(2):156-159. doi: 10.7774/cevr.2017.6.2.156.
Reference
-
1. Schwartz M. The life and works of Louis Pasteur. J Appl Microbiol. 2001; 91:597–601.
Article2. Girard MP, Osmanov SK, Kieny MP. A review of vaccine research and development: the human immunodeficiency virus (HIV). Vaccine. 2006; 24:4062–4081.
Article3. Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012; 64:1547–1568.
Article4. Dean CH, Alarcon JB, Waterston AM, et al. Cutaneous delivery of a live, attenuated chimeric flavivirus vaccine against Japanese encephalitis (ChimeriVax)-JE) in non-human primates. Hum Vaccin. 2005; 1:106–111.
Article5. Hirschberg HJ, van de Wijdeven GG, Kraan H, Amorij JP, Kersten GF. Bioneedles as alternative delivery system for hepatitis B vaccine. J Control Release. 2010; 147:211–217.
Article6. Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Enhanced memory responses to seasonal H1N1 influenza vaccination of the skin with the use of vaccine-coated microneedles. J Infect Dis. 2010; 201:190–198.
Article7. Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J Infect Dis. 2011; 204:582–591.
Article8. Kim YC, Quan FS, Yoo DG, Compans RW, Kang SM, Prausnitz MR. Improved influenza vaccination in the skin using vaccine coated microneedles. Vaccine. 2009; 27:6932–6938.
Article9. Quan FS, Kim YC, Yoo DG, Compans RW, Prausnitz MR, Kang SM. Stabilization of influenza vaccine enhances protection by microneedle delivery in the mouse skin. PLoS One. 2009; 4:e7152.
Article10. Kommareddy S, Baudner BC, Oh S, Kwon SY, Singh M, O'Hagan DT. Dissolvable microneedle patches for the delivery of cell-culture-derived influenza vaccine antigens. J Pharm Sci. 2012; 101:1021–1027.
Article11. Sullivan SP, Koutsonanos DG, Del Pilar Martin M, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010; 16:915–920.
Article12. Alarcon JB, Hartley AW, Harvey NG, Mikszta JA. Preclinical evaluation of microneedle technology for intradermal delivery of influenza vaccines. Clin Vaccine Immunol. 2007; 14:375–381.
Article13. Moon S, Wang Y, Edens C, Gentsch JR, Prausnitz MR, Jiang B. Dose sparing and enhanced immunogenicity of inactivated rotavirus vaccine administered by skin vaccination using a microneedle patch. Vaccine. 2013; 31:3396–3402.
Article14. del Pilar Martin M, Weldon WC, Zarnitsyn VG, et al. Local response to microneedle-based influenza immunization in the skin. MBio. 2012; 3:e00012-12.
Article15. Andrianov AK, DeCollibus DP, Gillis HA, et al. Poly[di(carboxylatophenoxy) phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci U S A. 2009; 106:18936–18941.
Article16. Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J Control Release. 2010; 142:187–195.
Article17. Weldon WC, Martin MP, Zarnitsyn V, et al. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin Vaccine Immunol. 2011; 18:647–654.
Article18. Fernando GJ, Chen X, Prow TW, et al. Potent immunity to low doses of influenza vaccine by probabilistic guided micro-targeted skin delivery in a mouse model. PLoS One. 2010; 5:e10266.
Article19. Raphael AP, Prow TW, Crichton ML, Chen X, Fernando GJ, Kendall MA. Targeted, needle-free vaccinations in skin using multilayered, densely-packed dissolving microprojection arrays. Small. 2010; 6:1785–1793.
Article20. Chen X, Corbett HJ, Yukiko SR, et al. Site-selectively coated, densely-packed microprojection array patches for targeted delivery of vaccines to skin. Adv Funct Mater. 2011; 21:464–473.
Article21. Koutsonanos DG, Vassilieva EV, Stavropoulou A, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep. 2012; 2:357.
Article22. Leroux-Roels I, Vets E, Freese R, et al. Seasonal influenza vaccine delivered by intradermal microinjection: a randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008; 26:6614–6619.
Article23. Beran J, Ambrozaitis A, Laiskonis A, et al. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009; 7:13.
Article24. Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009; 27:454–459.
Article25. Arnou R, Icardi G, De Decker M, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009; 27:7304–7312.
Article26. Corbett HJ, Fernando GJ, Chen X, Frazer IH, Kendall MA. Skin vaccination against cervical cancer associated human papillomavirus with a novel micro-projection array in a mouse model. PLoS One. 2010; 5:e13460.
Article27. Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation of microneedles coated with influenza virus-like particle vaccine. AAPS PharmSciTech. 2010; 11:1193–1201.
Article28. Kim YC, Quan FS, Song JM, et al. Influenza immunization with trehalose-stabilized virus-like particle vaccine using microneedles. Procedia Vaccinol. 2010; 2:15–19.
Article29. Quan FS, Kim YC, Vunnava A, et al. Intradermal vaccination with influenza virus-like particles by using microneedles induces protection superior to that with intramuscular immunization. J Virol. 2010; 84:7760–7769.
Article30. Song JM, Kim YC, Lipatov AS, et al. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin Vaccine Immunol. 2010; 17:1381–1389.
Article31. Song JM, Kim YC, Barlow PG, et al. Improved protection against avian influenza H5N1 virus by a single vaccination with virus-like particles in skin using microneedles. Antiviral Res. 2010; 88:244–247.
Article32. Quan FS, Kim YC, Compans RW, Prausnitz MR, Kang SM. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J Control Release. 2010; 147:326–332.
Article33. Pearton M, Kang SM, Song JM, et al. Influenza virus-like particles coated onto microneedles can elicit stimulatory effects on Langerhans cells in human skin. Vaccine. 2010; 28:6104–6113.
Article34. Mikszta JA, Sullivan VJ, Dean C, et al. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J Infect Dis. 2005; 191:278–288.
Article35. Wendorf JR, Ghartey-Tagoe EB, Williams SC, Enioutina E, Singh P, Cleary GW. Transdermal delivery of macromolecules using solid-state biodegradable microstructures. Pharm Res. 2011; 28:22–30.
Article36. Mikszta JA, Dekker JP 3rd, Harvey NG, et al. Microneedle-based intradermal delivery of the anthrax recombinant protective antigen vaccine. Infect Immun. 2006; 74:6806–6810.
Article37. Ding Z, Van Riet E, Romeijn S, Kersten GF, Jiskoot W, Bouwstra JA. Immune modulation by adjuvants combined with diphtheria toxoid administered topically in BALB/c mice after microneedle array pretreatment. Pharm Res. 2009; 26:1635–1643.
Article38. Ding Z, Verbaan FJ, Bivas-Benita M, et al. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J Control Release. 2009; 136:71–78.
Article39. Bal SM, Ding Z, Kersten GF, Jiskoot W, Bouwstra JA. Microneedle-based transcutaneous immunisation in mice with N-trimethyl chitosan adjuvanted diphtheria toxoid formulations. Pharm Res. 2010; 27:1837–1847.
Article40. Hirschberg HJ, van de Wijdeven GG, Kelder AB, van den Dobbelsteen GP, Kersten GF. Bioneedles as vaccine carriers. Vaccine. 2008; 26:2389–2397.
Article41. Hiraishi Y, Nandakumar S, Choi SO, et al. Bacillus Calmette-Guerin vaccination using a microneedle patch. Vaccine. 2011; 29:2626–2636.
Article42. Huang J, D'Souza AJ, Alarcon JB, et al. Protective immunity in mice achieved with dry powder formulation and alternative delivery of plague F1-V vaccine. Clin Vaccine Immunol. 2009; 16:719–725.
Article43. Morefield GL, Tammariello RF, Purcell BK, et al. An alternative approach to combination vaccines: intradermal administration of isolated components for control of anthrax, botulism, plague and staphylococcal toxic shock. J Immune Based Ther Vaccines. 2008; 6:5.
Article44. Prow TW, Chen X, Prow NA, et al. Nanopatch-targeted skin vaccination against West Nile Virus and Chikungunya virus in mice. Small. 2010; 6:1776–1784.
Article45. Gill HS, Soderholm J, Prausnitz MR, Sallberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010; 17:811–814.
Article46. Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002; 8:415–419.
Article47. Kask AS, Chen X, Marshak JO, et al. DNA vaccine delivery by densely-packed and short microprojection arrays to skin protects against vaginal HSV-2 challenge. Vaccine. 2010; 28:7483–7491.
Article48. Kumar A, Wonganan P, Sandoval MA, Li X, Zhu S, Cui Z. Microneedle-mediated transcutaneous immunization with plasmid DNA coated on cationic PLGA nanoparticles. J Control Release. 2012; 163:230–239.
Article49. DeMuth PC, Min Y, Huang B, et al. Polymer multilayer tattooing for enhanced DNA vaccination. Nat Mater. 2013; 12:367–376.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Microneedles: quick and easy delivery methods of vaccines
- Microneedle Transdermal Drug Delivery Systems for Allergen-Specific Immunotherapy, Skin Disease Treatment, and Vaccine Development
- Efficacy of Biodegradable Microneedle Patches on Periorbital Wrinkles
- Safety Evaluation of Stamp Type Digital Microneedle Devices in Hairless Mice
- Repeated Microneedle Stimulation Induces Enhanced Hair Growth in a Murine Model