Brain Tumor Res Treat.
2013 Oct;1(2):71-77. 10.14791/btrt.2013.1.2.71.
Bromocriptine Therapy for the Treatment of Invasive Prolactinoma: The Single Institute Experience
- Affiliations
-
- 1Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. shinhj@skku.edu
- KMID: 2048478
- DOI: http://doi.org/10.14791/btrt.2013.1.2.71
Abstract
OBJECTIVE
The objective of this study was to describe and characterize the clinical course of treatment for invasive prolactinoma patients using bromocriptine.
METHODS
The study group included 23 patients who were treated with bromocriptine for their invasive prolactinomas. Clinical histories, serum prolactin level and pituitary hormone assessments, tumor diameter and signal intensity on sella magnetic resonance imaging (MRI), visual field exams and the dosage of medications were reviewed for each patient.
RESULTS
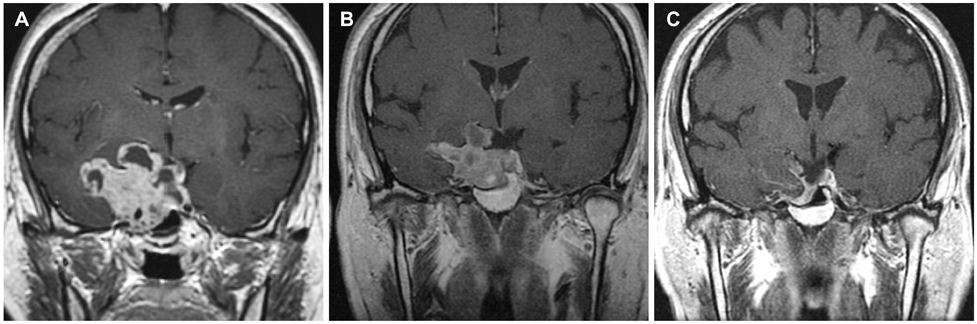
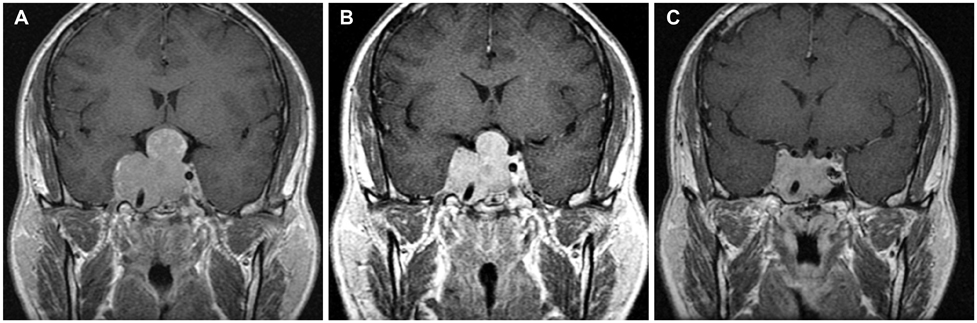
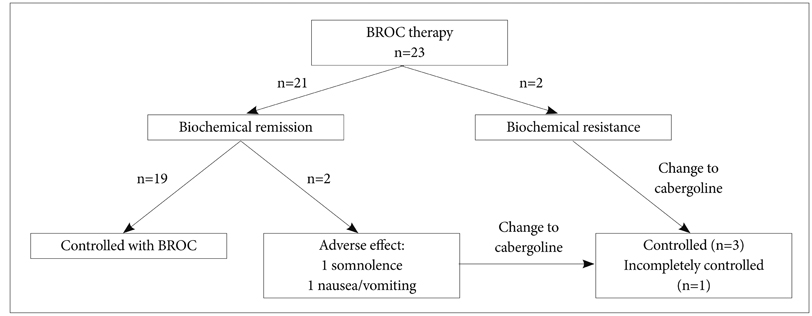
During 30 months (median, range 6-99) of follow-up period, 19 patients treated with bromocriptine alone achieved biochemical remission. Four patients changed the medication to cabergoline due to the adverse effects or observed resistance of bromocriptine treatment. All of five patients who had visual symptoms improved after the course of medication. Four surgically treated patients were not able to discontinue medication because they could not maintain biochemical remission state without medication. Multivariate analysis showed that decreased enhancement on the initial followed MRI after medication and longer follow-up periods were associated with higher radiologic response.
CONCLUSION
We reassure that the dopamine agonist is safe and effective for the treatment of invasive pituitary adenomas. Meanwhile, surgery has a limited role on biochemical remission. Decreased enhancement on the initial follow-up MRI after medication may reflect the treatment response. Further study is required to validate the role of MRI or other factors on the actual prognosis.
MeSH Terms
Figure
Reference
-
1. Schaller B. Gender-related differences in prolactinomas. A clinicopathological study. Neuro Endocrinol Lett. 2005; 26:152–159.2. Shimon I, Benbassat C, Hadani M. Effectiveness of long-term cabergoline treatment for giant prolactinoma: study of 12 men. Eur J Endocrinol. 2007; 156:225–231.
Article3. Wu ZB, Yu CJ, Su ZP, Zhuge QC, Wu JS, Zheng WM. Bromocriptine treatment of invasive giant prolactinomas involving the cavernous sinus: results of a long-term follow up. J Neurosurg. 2006; 104:54–61.
Article4. Fraioli MF, Novegno F, Catena E, Fraioli C, Moschettoni L. Multidisciplinary treatment of giant invasive prolactinomas in paediatric age: long-term follow-up in two children. Childs Nerv Syst. 2010; 26:1233–1237.
Article5. Serri O, Rasio E, Beauregard H, Hardy J, Somma M. Recurrence of hyperprolactinemia after selective transsphenoidal adenomectomy in women with prolactinoma. N Engl J Med. 1983; 309:280–283.
Article6. Nelson PB, Goodman M, Maroon JC, Martinez AJ, Moossy J, Robinson AG. Factors in predicting outcome from operation in patients with prolactin-secreting pituitary adenomas. Neurosurgery. 1983; 13:634–641.
Article7. Pinzone JJ, Katznelson L, Danila DC, Pauler DK, Miller CS, Klibanski A. Primary medical therapy of micro- and macroprolactinomas in men. J Clin Endocrinol Metab. 2000; 85:3053–3057.
Article8. Passos VQ, Souza JJ, Musolino NR, Bronstein MD. Long-term follow-up of prolactinomas: normoprolactinemia after bromocriptine withdrawal. J Clin Endocrinol Metab. 2002; 87:3578–3582.
Article9. Knosp E, Steiner E, Kitz K, Matula C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery. 1993; 33:610–617. discussion 617-8.
Article10. Molitch ME. Prolactin-secreting tumors: what's new? Expert Rev Anticancer Ther. 2006; 6:Suppl 9. S29–S35.
Article11. Saeki N, Nakamura M, Sunami K, Yamaura A. Surgical indication after bromocriptine therapy on giant prolactinomas: effects and limitations of the medical treatment. Endocr J. 1998; 45:529–537.
Article12. Wu ZB, Su ZP, Wu JS, Zheng WM, Zhuge QC, Zhong M. Five years follow-up of invasive prolactinomas with special reference to the control of cavernous sinus invasion. Pituitary. 2008; 11:63–70.
Article13. Colao A, Annunziato L, Lombardi G. Treatment of prolactinomas. Ann Med. 1998; 30:452–459.
Article14. Delgrange E, Duprez T, Maiter D. Influence of parasellar extension of macroprolactinomas defined by magnetic resonance imaging on their responsiveness to dopamine agonist therapy. Clin Endocrinol (Oxf). 2006; 64:456–462.
Article15. Cho EH, Lee SA, Chung JY, et al. Efficacy and safety of cabergoline as first line treatment for invasive giant prolactinoma. J Korean Med Sci. 2009; 24:874–878.
Article16. McCollough WM, Marcus RB Jr, Rhoton AL Jr, Ballinger WE, Million RR. Long-term follow-up of radiotherapy for pituitary adenoma: the absence of late recurrence after greater than or equal to 4500 cGy. Int J Radiat Oncol Biol Phys. 1991; 21:607–614.
Article17. Turbin RE, Thompson CR, Kennerdell JS, Cockerham KP, Kupersmith MJ. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology. 2002; 109:890–899. discussion 899-900.
Article18. Kontogeorgos G, Horvath E, Kovacs K, et al. Morphologic changes of prolactin-producing pituitary adenomas after short treatment with dopamine agonists. Acta Neuropathol. 2006; 111:46–52.
Article19. Yang MS, Hong JW, Lee SK, Lee EJ, Kim SH. Clinical management and outcome of 36 invasive prolactinomas treated with dopamine agonist. J Neurooncol. 2011; 104:195–204.
Article20. Lesser RL, Zheutlin JD, Boghen D, Odel JG, Robbins RJ. Visual function improvement in patients with macroprolactinomas treated with bromocriptine. Am J Ophthalmol. 1990; 109:535–543.
Article21. Zahra MA, Tan LT, Priest AN, et al. Semiquantitative and quantitative dynamic contrast-enhanced magnetic resonance imaging measurements predict radiation response in cervix cancer. Int J Radiat Oncol Biol Phys. 2009; 74:766–773.
Article22. George ML, Dzik-Jurasz AS, Padhani AR, et al. Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br J Surg. 2001; 88:1628–1636.
Article23. Toita T, Nakano M, Higashi M, Sakumoto K, Kanazawa K. Prognostic value of cervical size and pelvic lymph node status assessed by computed tomography for patients with uterine cervical cancer treated by radical radiation therapy. Int J Radiat Oncol Biol Phys. 1995; 33:843–849.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of SKP2 Sensitizes Bromocriptine-Induced Apoptosis in Human Prolactinoma Cells
- A Case of Pituitary Macroadenoma Concurrently Diagnosed in a Patient Undergoing Antipsychotic Treatment
- The Effect of Bromocriptine Treatment for Invasive Prolactinoma
- Comparison of Treatment Modalities in Hyperprolactinemia
- A Case of Bromocriptine Resistant Hyperprolactinemia Which was Responsive to Pergolide