Anat Cell Biol.
2013 Mar;46(1):68-78. 10.5115/acb.2013.46.1.68.
Expression of transient receptor potential channels in the ependymal cells of the developing rat brain
- Affiliations
-
- 1Department of Neurology, Gangneung Asan Hospital, University of Ulsan College of Medicine, Gangneung, Korea.
- 2Department of Anatomy, Kwandong University College of Medicine, Gangneung, Korea. dextrin@kd.ac.kr
- 3Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2046759
- DOI: http://doi.org/10.5115/acb.2013.46.1.68
Abstract
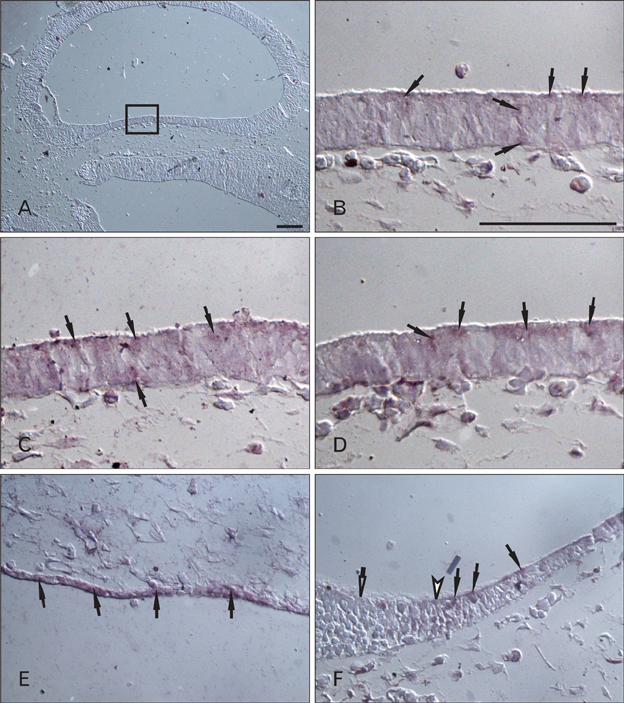
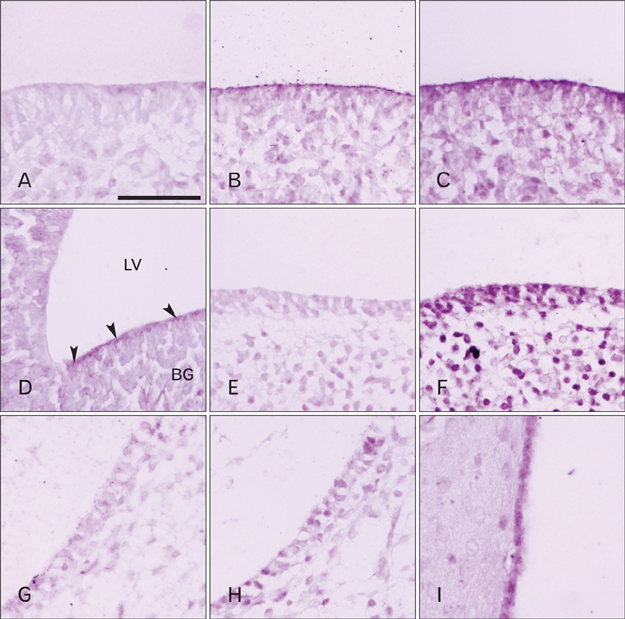
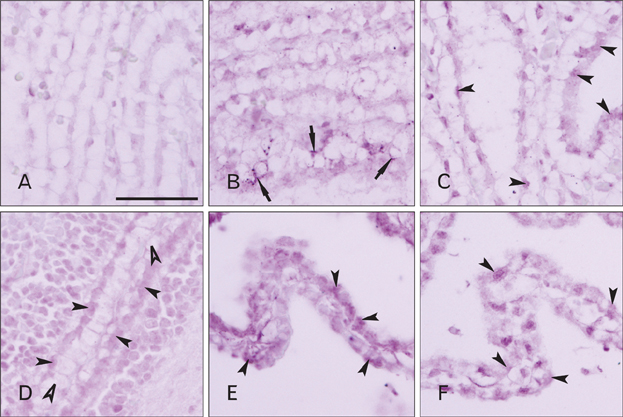
- Cerebrospinal fluid (CSF) plays an important role in providing brain tissue with a stable internal environment as well as in absorbing mechanical and thermal stresses. From its initial composition, derived from the amniotic fluid trapped by the closure of neuropores, CSF is modified by developing and differentiating ependymal cells lining the ventricular surface or forming the choroid plexus. Its osmolarity and ionic composition brings about a change through the action of many channels expressed on the ependymal cells. Some newly discovered transient receptor potential (TRP) channels are known to be expressed in the choroid plexus ependyma. To detect additional TRP channel expression, immunohistochemical screening was performed at the choroid plexus of 13-, 15-, 17-, and 19-day embryos, using antibodies against TRPV1, TRPV3, and TRPA1, and the expression was compared with those in the adult TRP channels. The level of TRP channel expression was higher in the choroid plexus which suggests more active functioning of TRP channels in the developing choroid plexus than the ventricular lining ependyma in the 15- and 17-day embryos. All the expression of TRP channels decreased at the 19th day of gestation. TRPA1 was expressed at a higher level than TRPV1 and TRPV3 in almost all stages in both the choroid plexus and ventricular lining epithelium. The highest level of TRPV1 and TRPV3 expression was observed in association with the glycogen deposits in the cytoplasm of the choroid plexus ependymal cells of the 15- and 17-day embryos.
MeSH Terms
Figure
Reference
-
1. Oldendorf WH, Davson H. Brain extracellular space and the sink action of cerebrospinal fluid. Measurement of rabbit brain extracellular space using sucrose labeled with carbon 14. Arch Neurol. 1967. 17:196–205.2. Di Terlizzi R, Platt S. The function, composition and analysis of cerebrospinal fluid in companion animals: part I - function and composition. Vet J. 2006. 172:422–431.3. Damkier HH, Brown PD, Praetorius J. Epithelial pathways in choroid plexus electrolyte transport. Physiology (Bethesda). 2010. 25:239–249.4. Millar ID, Bruce J, Brown PD. Ion channel diversity, channel expression and function in the choroid plexuses. Cerebrospinal Fluid Res. 2007. 4:8.5. Talavera K, Nilius B, Voets T. Neuronal TRP channels: thermometers, pathfinders and life-savers. Trends Neurosci. 2008. 31:287–295.6. Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000. 103:525–535.7. Harteneck C, Reiter B. TRP channels activated by extracellular hypo-osmoticity in epithelia. Biochem Soc Trans. 2007. 35(Pt 1):91–95.8. Benham CD, Davis JB, Randall AD. Vanilloid and TRP channels: a family of lipid-gated cation channels. Neuropharmacology. 2002. 42:873–888.9. Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005. 38:233–252.10. Voets T, Talavera K, Owsianik G, Nilius B. Sensing with TRP channels. Nat Chem Biol. 2005. 1:85–92.11. Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007. 76:387–417.12. Phelps CB, Wang RR, Choo SS, Gaudet R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J Biol Chem. 2010. 285:731–740.13. Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol. 2009. 75:1262–1279.14. Das GD. Gliogenesis and ependymogenesis during embryonic development of the rat. An autoradiographic study. J Neurol Sci. 1979. 43:193–204.15. Kilinc M, Deniz M, Ketani S, Hatipoglu ES. Prenatal and postnatal development of the rat choroid plexus. J Anim Vet Adv. 2010. 9:12–15.16. Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally deter mined patterns in the rat. Neurotoxicology. 1993. 14:83–144.17. Tennyson VM, Pappas GD. Fine structure of the developing telencephalic and myelencephalic choroid plexus in the rabbit. J Comp Neurol. 1964. 123:379–411.18. Dziegielewska KM, Ek J, Habgood MD, Saunders NR. Development of the choroid plexus. Microsc Res Tech. 2001. 52:5–20.19. Johansson PA, Dziegielewska KM, Ek CJ, Habgood MD, Møllgård K, Potter A, Schuliga M, Saunders NR. Aquaporin-1 in the choroid plexuses of developing mammalian brain. Cell Tissue Res. 2005. 322:353–364.20. de Rougemont , Ames A 3rd, Nesbett FB, Hofmann HF. Fluid formed by choroid plexus; a technique for its collection and a comparison of its electrolyte composition with serum and cisternal fluids. J Neurophysiol. 1960. 23:485–495.21. Oresković D, Klarica M. The formation of cerebrospinal fluid: nearly a hundred years of interpretations and misinterpretations. Brain Res Rev. 2010. 64:241–262.22. Liddelow SA, Temple S, Møllgård K, Gehwolf R, Wagner A, Bauer H, Bauer HC, Phoenix TN, Dziegielewska KM, Saunders NR. Molecular characterisation of transport mechanisms at the developing mouse blood-CSF interface: a transcriptome approach. PLoS One. 2012. 7:e33554.23. Goldstein MH, Bazer FW, Barron DH. Characterization of changes in volume, osmolarity and electrolyte composition of porcine fetal fluids during gestation. Biol Reprod. 1980. 22:1168–1180.24. Davson H, Purvis C. Cryoscopic apparatus suitable for studies on aqueous humour and cerebro-spinal fluid. J Physiol. 1954. 124:12P–13P.25. Ames A 3rd, Sakanoue M, Endo S. Na, K, Ca, Mg, and C1 concentrations in choroid plexus fluid and cisternal fluid compared with plasma ultrafiltrate. J Neurophysiol. 1964. 27:672–681.26. Hughes AL, Pakhomova A, Brown PD. Regulatory volume increase in epithelial cells isolated from the mouse fourth ventricle choroid plexus involves Na(+)-H(+) exchange but not Na(+)-K(+)-2Cl(-) cotransport. Brain Res. 2010. 1323:1–10.27. Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007. 87:165–217.28. Bayer SA, Altman J, Russo RJ, Dai XF, Simmons JA. Cell migration in the rat embryonic neocortex. J Comp Neurol. 1991. 307:499–516.29. Desmond ME, Jacobson AG. Embryonic brain enlargement requires cerebrospinal fluid pressure. Dev Biol. 1977. 57:188–198.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Transient Receptor Potential Channel in Pain
- Immunohistochemical Study on the Distribution of Canonical Transient Receptor Potential Channels in Rat Cerebellum
- Immunohistochemical Changes of Nestin, BrdU, and GFAP in the Cells of Rat Spinal Ependymal Zone According to Their Developmental Stages
- Identification of phospholipase C β downstream effect on transient receptor potential canonical 1/4, transient receptor potential canonical 1/5 channels
- Modulation of Dopaminergic Neuronal Excitability by Zinc through the Regulation of Calcium-related Channels