Anat Cell Biol.
2013 Mar;46(1):57-67. 10.5115/acb.2013.46.1.57.
Caffeine-induced endothelial cell death and the inhibition of angiogenesis
- Affiliations
-
- 1Department of Anatomy, Chungbuk National University Medical School, Cheongju, Korea.
- 2Department of Hematology, Affiliated Hospital of Yanbian University, Yanji, China.
- 3Department of Anatomy and Cell Biology, Gachon University School of Medicine, Incheon, Korea. gbjeong@gachon.ac.kr
- KMID: 2046758
- DOI: http://doi.org/10.5115/acb.2013.46.1.57
Abstract
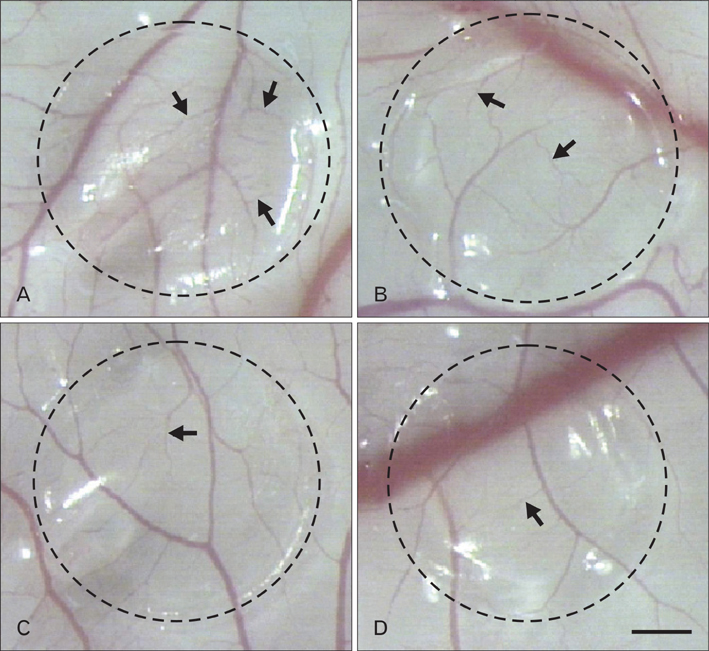
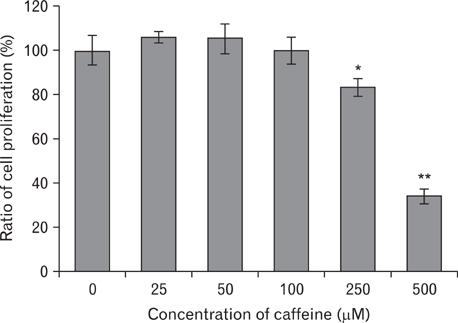
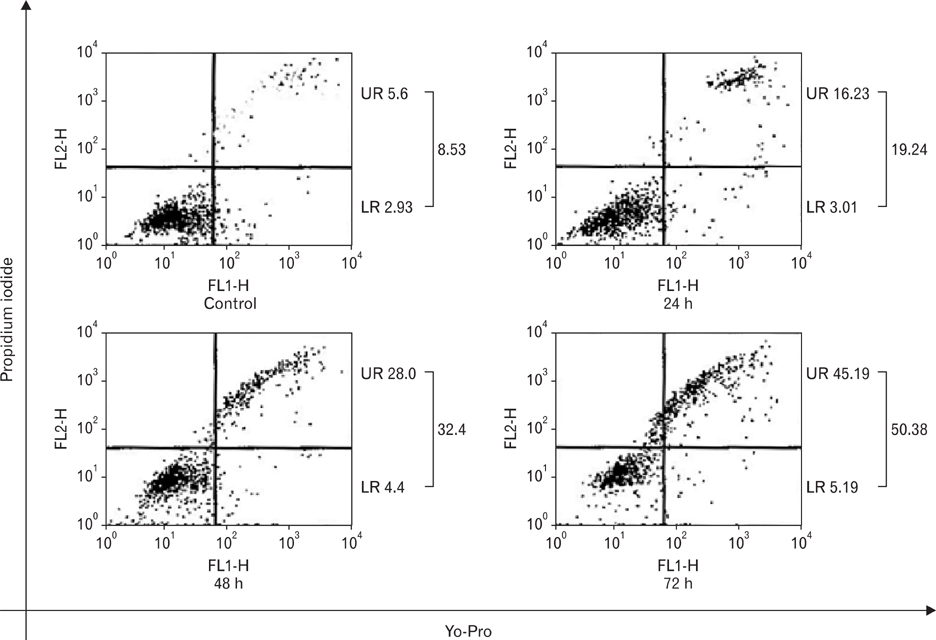
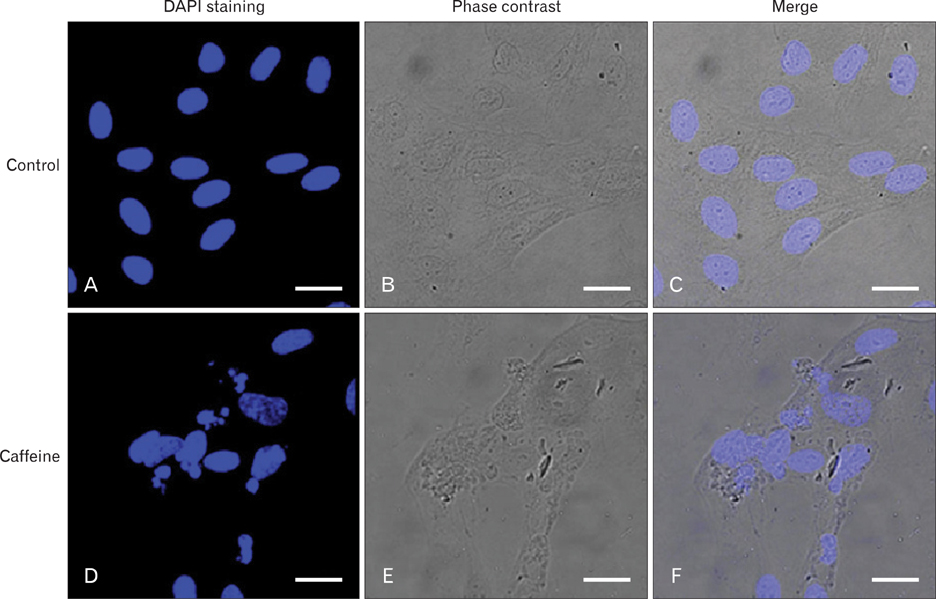
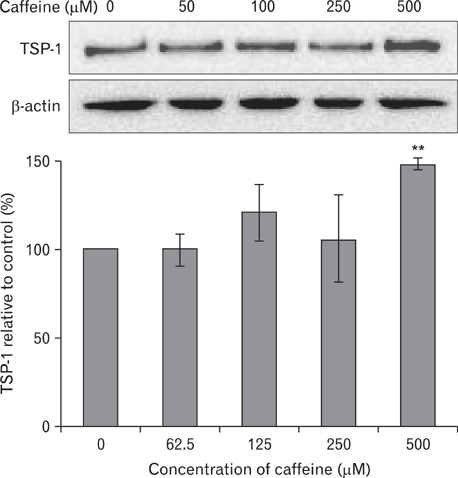
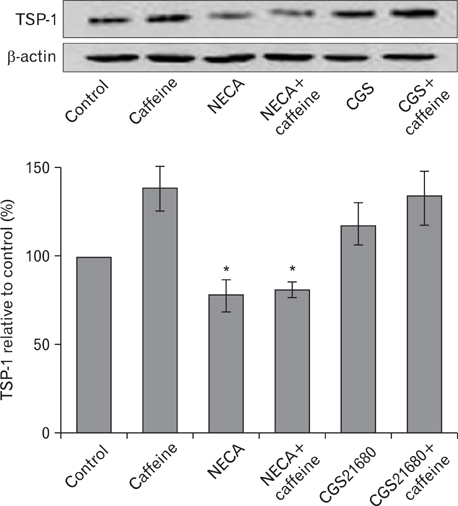
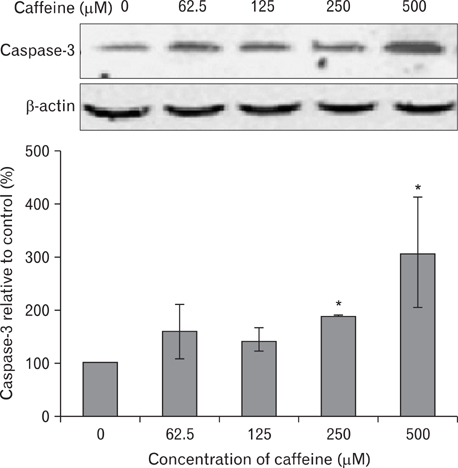
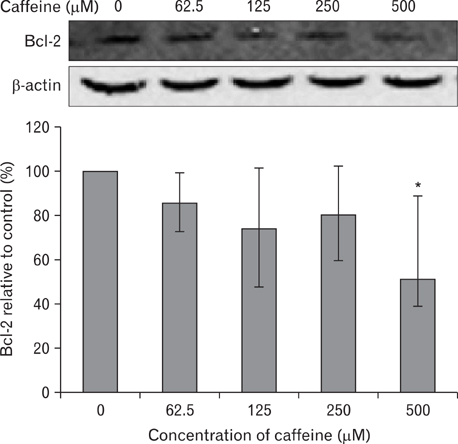
- Numerous studies have shown that adenosine or adenosine agonists can stimulate angiogenesis. However, the effect of caffeine (a known adenosine receptor antagonist) on angiogenesis has not been previously studied. Accordingly, this study was undertaken to examine the effect of caffeine on angiogenesis and to clarify the mechanism involved. Chick chorioallantoic membrane assays were used to investigate the effect of caffeine on angiogenesis and proliferation assays using human umbilical vein endothelial cells (HUVECs), were used to study its effects on specific aspects of angiogenesis. The expressions of caspase-3 and Bcl-2 were examined by western blotting, immunofluorescence staining was used to identify HUVEC morphological changes, and fluorescence activated cell sorting (FACS) and DAPI staining were used to detect HUVEC apoptosis. Caffeine was found to inhibit blood vessel formation dose-dependently and to inhibit the proliferation of HUVECs time- and dose-dependently. FACS analysis and DAPI staining showed that inhibitory effect of caffeine on HUVEC proliferation was the result of apoptosis and the up-regulation of thrombospondin-1 (TSP-1). Furthermore, TSP-1 levels were down-regulated by NECA but were unaffected by CGS21680, indicating that caffeine regulated TSP-1 expression via adenosine A2B receptor. In addition, caffeine up-regulated caspase-3 and down-regulated Bcl-2 at the protein level. These results suggest that the inhibitory effect of caffeine on angiogenesis is associated, at least in part, with its induction of endothelial cell apoptosis, probably mediated by a caspase-3 dependent mechanism.
MeSH Terms
-
Adenosine
Adenosine-5'-(N-ethylcarboxamide)
Apoptosis
Blood Vessels
Blotting, Western
Caffeine
Caspase 3
Chorioallantoic Membrane
Endothelial Cells
Flow Cytometry
Fluorescent Antibody Technique
Glycosaminoglycans
Human Umbilical Vein Endothelial Cells
Indoles
Phenethylamines
Receptor, Adenosine A2B
Receptors, Purinergic P1
Thrombospondin 1
Up-Regulation
Adenosine
Adenosine-5'-(N-ethylcarboxamide)
Caffeine
Caspase 3
Glycosaminoglycans
Indoles
Phenethylamines
Receptor, Adenosine A2B
Receptors, Purinergic P1
Thrombospondin 1
Figure
Reference
-
1. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995. 1:27–31.2. Yeh CH, Liao YF, Chang CY, Tsai JN, Wang YH, Cheng CC, Wen CC, Chen YH. Caffeine treatment disturbs the angiogenesis of zebrafish embryos. Drug Chem Toxicol. 2012. 35:361–365.3. Hildebrand JS, Patel AV, McCullough ML, Gaudet MM, Chen AY, Hayes RB, Gapstur SM. Coffee, tea, and fatal oral/pharyngeal cancer in a large prospective US cohort. Am J Epidemiol. 2013. 177:50–58.4. Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, Cronstein BN. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol. 2002. 160:2009–2018.5. Montesinos MC, Desai A, Delano D, Chen JF, Fink JS, Jacobson MA, Cronstein BN. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum. 2003. 48:240–247.6. Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997. 186:1615–1620.7. Montesinos MC, Yap JS, Desai A, Posadas I, McCrary CT, Cronstein BN. Reversal of the antiinflammatory effects of methotrexate by the nonselective adenosine receptor antagonists theophylline and caffeine: evidence that the antiinflammatory effects of methotrexate are mediated via multiple adenosine receptors in rat adjuvant arthritis. Arthritis Rheum. 2000. 43:656–663.8. Victor-Vega C, Desai A, Montesinos MC, Cronstein BN. Adenosine A2A receptor agonists promote more rapid wound healing than recombinant human platelet-derived growth factor (Becaplermin gel). Inflammation. 2002. 26:19–24.9. Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res. 2002. 90:531–538.10. Gu JW, Brady AL, Anand V, Moore MC, Kelly WC, Adair TH. Adenosine upregulates VEGF expression in cultured myocardial vascular smooth muscle cells. Am J Physiol. 1999. 277(2 Pt 2):H595–H602.11. Hashimoto E, Kage K, Ogita T, Nakaoka T, Matsuoka R, Kira Y. Adenosine as an endogenous mediator of hypoxia for induction of vascular endothelial growth factor mRNA in U-937 cells. Biochem Biophys Res Commun. 1994. 204:318–324.12. Leibovich SJ, Chen JF, Pinhal-Enfield G, Belem PC, Elson G, Rosania A, Ramanathan M, Montesinos C, Jacobson M, Schwarzschild MA, Fink JS, Cronstein B. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A(2A) receptor agonists and endotoxin. Am J Pathol. 2002. 160:2231–2244.13. Pueyo ME, Chen Y, D'Angelo G, Michel JB. Regulation of vascular endothelial growth factor expression by cAMP in rat aortic smooth muscle cells. Exp Cell Res. 1998. 238:354–358.14. Takagi H, King GL, Robinson GS, Ferrara N, Aiello LP. Adenosine mediates hypoxic induction of vascular endothelial growth factor in retinal pericytes and endothelial cells. Invest Ophthalmol Vis Sci. 1996. 37:2165–2176.15. Desai A, Victor-Vega C, Gadangi S, Montesinos MC, Chu CC, Cronstein BN. Adenosine A2A receptor stimulation increases angiogenesis by down-regulating production of the antiangiogenic matrix protein thrombospondin 1. Mol Pharmacol. 2005. 67:1406–1413.16. Adolfsson J. The time dependence of training-induced increase in skeletal muscle capillarization and the spatial capillary to fibre relationship in normal and neovascularized skeletal muscle of rats. Acta Physiol Scand. 1986. 128:259–266.17. Tornling G, Adolfsson J, Unge G, Ljungqvist A. Capillary neoformation in skeletal muscle of dipyridamole-treated rats. Arzneimittelforschung. 1980. 30:791–792.18. Tornling G. Capillary neoformation in the heart of dipyridamole-treated rats. Acta Pathol Microbiol Immunol Scand A. 1982. 90:269–271.19. Wothe D, Hohimer A, Morton M, Thornburg K, Giraud G, Davis L. Increased coronary blood flow signals growth of coronary resistance vessels in near-term ovine fetuses. Am J Physiol Regul Integr Comp Physiol. 2002. 282:R295–R302.20. Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, Franklin WA, Bridges JP, Schaack JB, Colgan SP, White CW. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci U S A. 2009. 106:10684–10689.21. Dusseau JW, Hutchins PM, Malbasa DS. Stimulation of angiogenesis by adenosine on the chick chorioallantoic membrane. Circ Res. 1986. 59:163–170.22. Fenselau A. An angiogenic role for adenine nucleotide catabolites. Fed Proc. 1984. 43:587.23. Jen SC, Rovainen CM. An adenosine agonist increases blood flow and density of capillary branches in the optic tectum of Xenopus laevis tadpoles. Microcirculation. 1994. 1:59–66.24. Cronstein BN. Adenosine receptors and wound healing. ScientificWorldJournal. 2004. 4:1–8.25. Roberts DD. Regulation of tumor growth and metastasis by thrombospondin-1. FASEB J. 1996. 10:1183–1191.26. Tuszynski GP, Nicosia RF. The role of thrombospondin-1 in tumor progression and angiogenesis. Bioessays. 1996. 18:71–76.27. Bornstein P. Thrombospondins function as regulators of angiogenesis. J Cell Commun Signal. 2009. 3:189–200.28. Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003. 22:63–71.29. Nor JE, Mitra RS, Sutorik MM, Mooney DJ, Castle VP, Polverini PJ. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J Vasc Res. 2000. 37:209–218.30. Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000. 12:634–640.31. Volpert OV. Modulation of endothelial cell survival by an inhibitor of angiogenesis thrombospondin-1: a dynamic balance. Cancer Metastasis Rev. 2000. 19:87–92.32. Jin RJ, Kwak C, Lee SG, Lee CH, Soo CG, Park MS, Lee E, Lee SE. The application of an anti-angiogenic gene (thrombospondin-1) in the treatment of human prostate cancer xenografts. Cancer Gene Ther. 2000. 7:1537–1542.33. Yamaguchi M, Sugio K, Ondo K, Yano T, Sugimachi K. Reduced expression of thrombospondin-1 correlates with a poor prognosis in patients with non-small cell lung cancer. Lung Cancer. 2002. 36:143–150.34. Tanaka K, Sonoo H, Kurebayashi J, Nomura T, Ohkubo S, Yamamoto Y, Yamamoto S. Inhibition of infiltration and angiogenesis by thrombospondin-1 in papillary thyroid carcinoma. Clin Cancer Res. 2002. 8:1125–1131.35. Garside SA, Harlow CR, Hillier SG, Fraser HM, Thomas FH. Thrombospondin-1 inhibits angiogenesis and promotes follicular atresia in a novel in vitro angiogenesis assay. Endocrinology. 2010. 151:1280–1289.36. Rege TA, Stewart J Jr, Dranka B, Benveniste EN, Silverstein RL, Gladson CL. Thrombospondin-1-induced apoptosis of brain microvascular endothelial cells can be mediated by TNF-R1. J Cell Physiol. 2009. 218:94–103.37. Lemus D, Dabancens A, Illanes J, Fuenzalida M, Guerrero A, López C. Antiangiogenic effect of betamethasone on the chick cam stimulated by TA3 tumor supernatant. Biol Res. 2001. 34:227–236.38. Xiao F, Wei Y, Yang L, Zhao X, Tian L, Ding Z, Yuan S, Lou Y, Liu F, Wen Y, Li J, Deng H, Kang B, Mao Y, Lei S, He Q, Su J, Lu Y, Niu T, Hou J, Huang MJ. A gene therapy for cancer based on the angiogenesis inhibitor, vasostatin. Gene Ther. 2002. 9:1207–1213.39. Blebea J, Mazo JE, Kihara TK, Vu JH, McLaughlin PJ, Atnip RG, Zagon IS. Opioid growth factor modulates angiogenesis. J Vasc Surg. 2000. 32:364–373.40. Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins: identification by morphologic and immunologic criteria. J Clin Invest. 1973. 52:2745–2756.41. Smeets EF, von Asmuth EJ, van der Linden CJ, Leeuwenberg JF, Buurman WA. A comparison of substrates for human umbilical vein endothelial cell culture. Biotech Histochem. 1992. 67:241–250.42. Balcerczyk A, Soszynski M, Rybaczek D, Przygodzki T, Karowicz-Bilinska A, Maszewski J, Bartosz G. Induction of apoptosis and modulation of production of reactive oxygen species in human endothelial cells by diphenyleneiodonium. Biochem Pharmacol. 2005. 69:1263–1273.43. Lou YR, Lu YP, Xie JG, Huang MT, Conney AH. Effects of oral administration of tea, decaffeinated tea, and caffeine on the formation and growth of tumors in high-risk SKH-1 mice previously treated with ultraviolet B light. Nutr Cancer. 1999. 33:146–153.44. Jacquet P, de Saint-Georges L, Barrio S, Baugnet-Mahieu L. Morphological effects of caffeine, okadaic acid and genistein in one-cell mouse embryos blocked in G2 by X-irradiation. Int J Radiat Biol. 1995. 67:347–358.45. Hashimoto T, He Z, Ma WY, Schmid PC, Bode AM, Yang CS, Dong Z. Caffeine inhibits cell proliferation by G0/G1 phase arrest in JB6 cells. Cancer Res. 2004. 64:3344–3349.46. Ito K, Nakazato T, Miyakawa Y, Yamato K, Ikeda Y, Kizaki M. Caffeine induces G2/M arrest and apoptosis via a novel p53-dependent pathway in NB4 promyelocytic leukemia cells. J Cell Physiol. 2003. 196:276–283.47. Fisone G, Borgkvist A, Usiello A. Caffeine as a psychomotor stimulant: mechanism of action. Cell Mol Life Sci. 2004. 61:857–872.48. Bode AM, Dong Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett. 2007. 247:26–39.49. Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999. 59:4375–4382.50. Saiki S, Sasazawa Y, Imamichi Y, Kawajiri S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa K, Imoto M, Hattori N. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy. 2011. 7:176–187.51. Kuwayama H. Arachidonic acid enhances caffeine-induced cell death via caspase-independent cell death. Sci Rep. 2012. 2:577.52. Gururajanna B, Al-Katib AA, Li YW, Aranha O, Vaitkevicius VK, Sarkar FH. Molecular effects of taxol and caffeine on pancreatic cancer cells. Int J Mol Med. 1999. 4:501–507.53. Qi W, Qiao D, Martinez JD. Caffeine induces TP53-independent G(1)-phase arrest and apoptosis in human lung tumor cells in a dose-dependent manner. Radiat Res. 2002. 157:166–174.54. He Z, Ma WY, Hashimoto T, Bode AM, Yang CS, Dong Z. Induction of apoptosis by caffeine is mediated by the p53, Bax, and caspase 3 pathways. Cancer Res. 2003. 63:4396–4401.55. Grant MB, Davis MI, Caballero S, Feoktistov I, Biaggioni I, Belardinelli L. Proliferation, migration, and ERK activation in human retinal endothelial cells through A(2B) adenosine receptor stimulation. Invest Ophthalmol Vis Sci. 2001. 42:2068–2073.56. Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I. Mast cell-mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ Res. 2003. 92:485–492.57. Afzal A, Shaw LC, Caballero S, Spoerri PE, Lewin AS, Zeng D, Belardinelli L, Grant MB. Reduction in preretinal neovascularization by ribozymes that cleave the A2B adenosine receptor mRNA. Circ Res. 2003. 93:500–506.58. Nguyen DK, Montesinos MC, Williams AJ, Kelly M, Cronstein BN. Th1 cytokines regulate adenosine receptors and their downstream signaling elements in human microvascular endothelial cells. J Immunol. 2003. 171:3991–3998.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Angiogenesis by the First Type I Repeat Peptides of Thrombospondin-1
- Thrombospondin-1 and Inhibition of Tumor Growth
- Observation of Endothelial Cell Differentiation by 3D Cell culture system: Angiogenesis inhibition of Thrombospondin-1
- Hypoxia in Hepatocellular Carcinoma
- Effect of caffeine on the Ca2+ pool affecting contractility and actomyosin ATPase activity in vascular smooth muscle of rabbit