Anat Cell Biol.
2013 Mar;46(1):19-31. 10.5115/acb.2013.46.1.19.
Molecular regulation of kidney development
- Affiliations
-
- 1Department of Anatomy, Institute for Medical Sciences, Chonbuk National University Medical School, Jeonju, Korea.
- 2Biomedical Research Institute, Chonbuk National University Medical School, Jeonju, Korea. oasis@jbnu.ac.kr, kwon@jbnu.ac.kr
- 3Department of Internal Medicine, Research Institute of Clinical Medicine, Chonbuk National University Medical School, Jeonju, Korea.
- 4Laboratory for Craniofacial Biology, Institute of Oral Biosciences, Chonbuk National University, School of Dentistry, Jeonju, Korea.
- KMID: 2046754
- DOI: http://doi.org/10.5115/acb.2013.46.1.19
Abstract
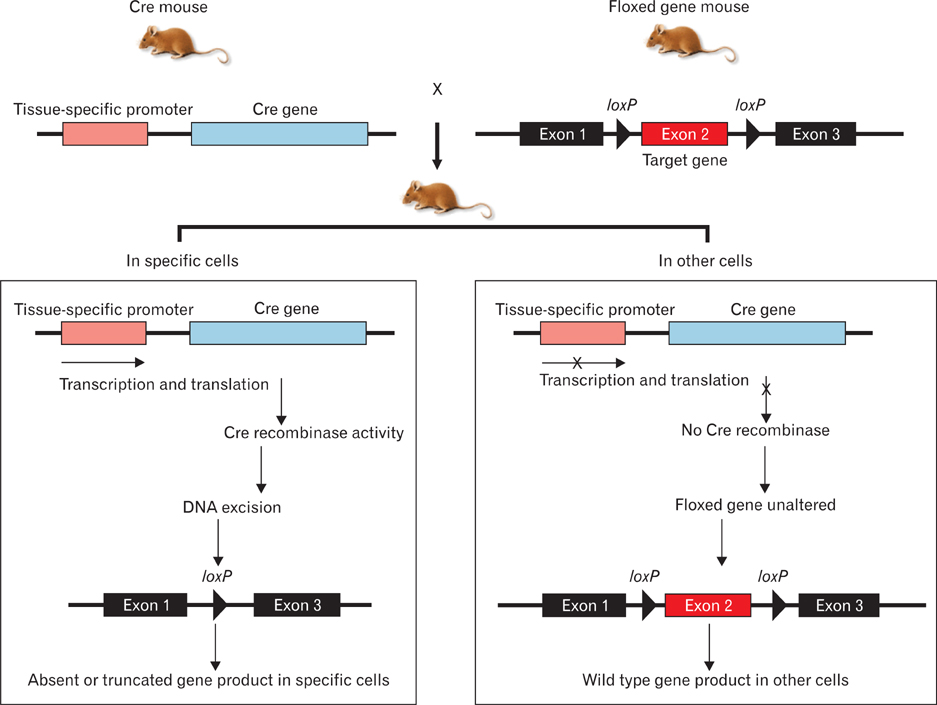
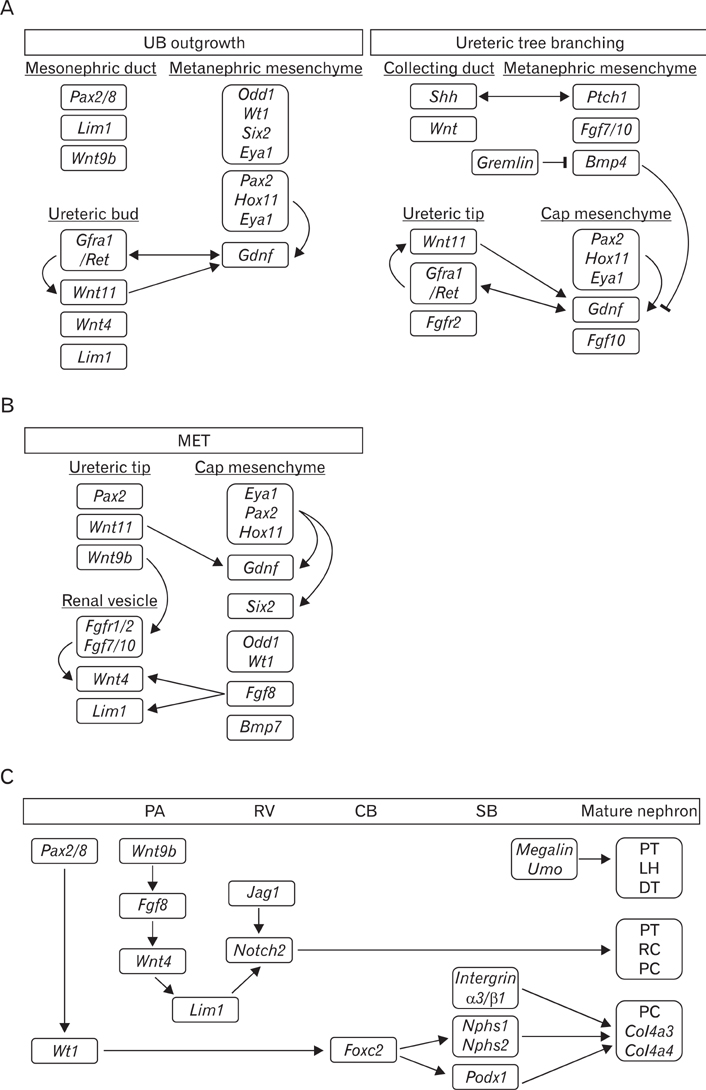
- Genetically engineered mice have provided much information about gene function in the field of developmental biology. Recently, conditional gene targeting using the Cre/loxP system has been developed to control the cell type and timing of the target gene expression. The increase in number of kidney-specific Cre mice allows for the analysis of phenotypes that cannot be addressed by conventional gene targeting. The mammalian kidney is a vital organ that plays a critical homeostatic role in the regulation of body fluid composition and excretion of waste products. The interactions between epithelial and mesenchymal cells are very critical events in the field of developmental biology, especially renal development. Kidney development is a complex process, requiring inductive interactions between epithelial and mesenchymal cells that eventually lead to the growth and differentiation of multiple highly specialized stromal, vascular, and epithelial cell types. Through the use of genetically engineered mouse models, the molecular bases for many of the events in the developing kidney have been identified. Defective morphogenesis may result in clinical phenotypes that range from complete renal agenesis to diseases such as hypertension that exist in the setting of grossly normal kidneys. In this review, we focus on the growth and transcription factors that define kidney progenitor cell populations, initiate ureteric bud branching, induce nephron formation within the metanephric mesenchyme, and differentiate stromal and vascular progenitors in the metanephric mesenchyme.
MeSH Terms
-
Animals
Body Fluids
Congenital Abnormalities
Developmental Biology
Epithelial Cells
Gene Expression
Gene Targeting
Hypertension
Kidney
Kidney Diseases
Mesoderm
Mice
Morphogenesis
Nephrons
Phenotype
Stem Cells
Transcription Factors
Ureter
Waste Products
Congenital Abnormalities
Kidney
Kidney Diseases
Transcription Factors
Waste Products
Figure
Cited by 1 articles
-
Exploration of the fetus with gross anomaly: a case of pseudo prune belly syndrome
Bhagyam Valappil, Lalu Krishna, Ranjith Sreedharan, Ashwija Shetty
Anat Cell Biol. 2018;51(3):205-208. doi: 10.5115/acb.2018.51.3.205.
Reference
-
1. Faa G, Gerosa C, Fanni D, Monga G, Zaffanello M, Van Eyken P, Fanos V. Morphogenesis and molecular mechanisms involved in human kidney development. J Cell Physiol. 2012. 227:1257–1268.2. Kohan DE. Progress in gene targeting: using mutant mice to study renal function and disease. Kidney Int. 2008. 74:427–437.3. Little M, Georgas K, Pennisi D, Wilkinson L. Kidney development: two tales of tubulogenesis. Curr Top Dev Biol. 2010. 90:193–229.4. Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol. 1981. 150:467–486.5. Wu F. Conditional targeting in the kidney. Nephron Physiol. 2007. 107:p10–p16.6. Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, Breton S, Nelson RD. V-ATPase B1-subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol. 2005. 288:C1134–C1144.7. Saxén L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987. 1:385–392.8. Hoch M, Broadie K, Jäckle H, Skaer H. Sequential fates in a single cell are established by the neurogenic cascade in the Malpighian tubules of Drosophila. Development. 1994. 120:3439–3450.9. Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012. 4:pii: a008300.10. Reidy KJ, Rosenblum ND. Cell and molecular biology of kidney development. Semin Nephrol. 2009. 29:321–337.11. Kuure S, Vuolteenaho R, Vainio S. Kidney morphogenesis: cellular and molecular regulation. Mech Dev. 2000. 92:31–45.12. Bouchard M. Transcriptional control of kidney development. Differentiation. 2004. 72:295–306.13. Ribes D, Fischer E, Calmont A, Rossert J. Transcriptional control of epithelial differentiation during kidney development. J Am Soc Nephrol. 2003. 14:Suppl 1. S9–S15.14. Barak H, Rosenfelder L, Schultheiss TM, Reshef R. Cell fate specification along the anterior-posterior axis of the intermediate mesoderm. Dev Dyn. 2005. 232:901–914.15. Mudumana SP, Hentschel D, Liu Y, Vasilyev A, Drummond IA. Odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate. Development. 2008. 135:3355–3367.16. James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006. 133:2995–3004.17. Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R. Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol. 2005. 288:582–594.18. Sajithlal G, Zou D, Silvius D, Xu PX. Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol. 2005. 284:323–336.19. Cirio MC, Hui Z, Haldin CE, Cosentino CC, Stuckenholz C, Chen X, Hong SK, Dawid IB, Hukriede NA. Lhx1 is required for specification of the renal progenitor cell field. PLoS One. 2011. 6:e18858.20. Sato A, Matsumoto Y, Koide U, Kataoka Y, Yoshida N, Yokota T, Asashima M, Nishinakamura R. Zinc finger protein sall2 is not essential for embryonic and kidney development. Mol Cell Biol. 2003. 23:62–69.21. Brodbeck S, Besenbeck B, Englert C. The transcription factor Six2 activates expression of the Gdnf gene as well as its own promoter. Mech Dev. 2004. 121:1211–1222.22. Tsang TE, Shawlot W, Kinder SJ, Kobayashi A, Kwan KM, Schughart K, Kania A, Jessell TM, Behringer RR, Tam PP. Lim1 activity is required for intermediate mesoderm differentiation in the mouse embryo. Dev Biol. 2000. 223:77–90.23. Gong KQ, Yallowitz AR, Sun H, Dressler GR, Wellik DM. A Hox-Eya-Pax complex regulates early kidney developmental gene expression. Mol Cell Biol. 2007. 27:7661–7668.24. Brodbeck S, Englert C. Genetic determination of nephrogenesis: the Pax/Eya/Six gene network. Pediatr Nephrol. 2004. 19:249–255.25. Davies JA, Fisher CE. Genes and proteins in renal development. Exp Nephrol. 2002. 10:102–113.26. Chai L. The role of HSAL (SALL) genes in proliferation and differentiation in normal hematopoiesis and leukemogenesis. Transfusion. 2011. 51:Suppl 4. 87S–93S.27. Nystrom J, Hultenby K, Ek S, Sjölund J, Axelson H, Jirström K, Saleem MA, Nilsson K, Johansson ME. CRIM1 is localized to the podocyte filtration slit diaphragm of the adult human kidney. Nephrol Dial Transplant. 2009. 24:2038–2044.28. Iwata T, Miyata Y, Kanda S, Nishikido M, Hayashi T, Sakai H, Kanetake H. Lymphangiogenesis and angiogenesis in conventional renal cell carcinoma: association with vascular endothelial growth factors A to D immunohistochemistry. Urology. 2008. 71:749–754.29. Nikopoulos GN, Martins JF, Adams TL, Karaczyn A, Adams D, Vary C, Oxburgh L, Verdi JM. NRAGE: a potential rheostat during branching morphogenesis. Mech Dev. 2009. 126:337–349.30. Marty MS, Neeper-Bradley TL, Neptun DA, Carney EW. Developmental toxicity of diethanolamine applied cutaneously to CD rats and New Zealand White rabbits. Regul Toxicol Pharmacol. 1999. 30:169–181.31. Benz K, Campean V, Cordasic N, Karpe B, Neuhuber W, Mall G, Hartner A, Hilgers KF, Amann K. Early glomerular alterations in genetically determined low nephron number. Am J Physiol Renal Physiol. 2011. 300:F521–F530.32. Fukushi Y, Orikasa S, Shepard T, Hakomori S. Changes of Lex and dimeric Lex haptens and their sialylated antigens during development of human kidney and kidney tumors. J Urol. 1986. 135:1048–1056.33. Jain S. The many faces of RET dysfunction in kidney. Organogenesis. 2009. 5:95–108.34. Shah MM, Sakurai H, Sweeney DE, Gallegos TF, Bush KT, Esko JD, Nigam SK. Hs2st mediated kidney mesenchyme induction regulates early ureteric bud branching. Dev Biol. 2010. 339:354–365.35. Cain JE, Rosenblum ND. Control of mammalian kidney development by the Hedgehog signaling pathway. Pediatr Nephrol. 2011. 26:1365–1371.36. Hu MC, Mo R, Bhella S, Wilson CW, Chuang PT, Hui CC, Rosenblum ND. GLI3-dependent transcriptional repression of Gli1, Gli2 and kidney patterning genes disrupts renal morphogenesis. Development. 2006. 133:569–578.37. Yu J, Carroll TJ, McMahon AP. Sonic hedgehog regulates proliferation and differentiation of mesenchymal cells in the mouse metanephric kidney. Development. 2002. 129:5301–5312.38. Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001. 187:265–276.39. Oxburgh L, Chu GC, Michael SK, Robertson EJ. TGFbeta superfamily signals are required for morphogenesis of the kidney mesenchyme progenitor population. Development. 2004. 131:4593–4605.40. Bracken CM, Mizeracka K, McLaughlin KA. Patterning the embryonic kidney: BMP signaling mediates the differentiation of the pronephric tubules and duct in Xenopus laevis. Dev Dyn. 2008. 237:132–144.41. Godin RE, Robertson EJ, Dudley AT. Role of BMP family members during kidney development. Int J Dev Biol. 1999. 43:405–411.42. Carev D, Saraga M, Saraga-Babic M. Involvement of FGF and BMP family proteins and VEGF in early human kidney development. Histol Histopathol. 2008. 23:853–862.43. Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004. 131:3401–3410.44. Hartwig S, Hu MC, Cella C, Piscione T, Filmus J, Rosenblum ND. Glypican-3 modulates inhibitory Bmp2-Smad signaling to control renal development in vivo. Mech Dev. 2005. 122:928–938.45. Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005. 16:139–149.46. Bates CM. Role of fibroblast growth factor receptor signaling in kidney development. Am J Physiol Renal Physiol. 2011. 301:F245–F251.47. Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol. 2006. 291:325–339.48. Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol. 2004. 276:403–415.49. Saifudeen Z, Dipp S, Stefkova J, Yao X, Lookabaugh S, El-Dahr SS. p53 regulates metanephric development. J Am Soc Nephrol. 2009. 20:2328–2337.50. Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol. 2008. 313:234–245.51. Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005. 9:283–292.52. Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008. 3:169–181.53. Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G. Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J. 2006. 25:5214–5228.54. Park JS, Ma W, O'Brien LL, Chung E, Guo JJ, Cheng JG, Valerius MT, McMahon JA, Wong WH, McMahon AP. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell. 2012. 23:637–651.55. Kreidberg JA. WT1 and kidney progenitor cells. Organogenesis. 2010. 6:61–70.56. Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993. 74:679–691.57. Fanni D, Fanos V, Monga G, Gerosa C, Locci A, Nemolato S, Van Eyken P, Faa G. Expression of WT1 during normal human kidney development. J Matern Fetal Neonatal Med. 2011. 24:Suppl 2. 44–47.58. Gai Z, Zhou G, Itoh S, Morimoto Y, Tanishima H, Hatamura I, Uetani K, Ito M, Muragaki Y. Trps1 functions downstream of Bmp7 in kidney development. J Am Soc Nephrol. 2009. 20:2403–2411.59. Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995. 9:2795–2807.60. Kazama I, Mahoney Z, Miner JH, Graf D, Economides AN, Kreidberg JA. Podocyte-derived BMP7 is critical for nephron development. J Am Soc Nephrol. 2008. 19:2181–2191.61. Perantoni AO, Timofeeva O, Naillat F, Richman C, Pajni-Underwood S, Wilson C, Vainio S, Dove LF, Lewandoski M. Inactivation of FGF8 in early mesoderm reveals an essential role in kidney development. Development. 2005. 132:3859–3871.62. Grieshammer U, Cebrián C, Ilagan R, Meyers E, Herzlinger D, Martin GR. FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development. 2005. 132:3847–3857.63. Hendry C, Rumballe B, Moritz K, Little MH. Defining and redefining the nephron progenitor population. Pediatr Nephrol. 2011. 26:1395–1406.64. Brunskill EW, Aronow BJ, Georgas K, Rumballe B, Valerius MT, Aronow J, Kaimal V, Jegga AG, Yu J, Grimmond S, McMahon AP, Patterson LT, Little MH, Potter SS. Atlas of gene expression in the developing kidney at microanatomic resolution. Dev Cell. 2008. 15:781–791.65. Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998. 125:4225–4234.66. Park JS, Valerius MT, McMahon AP. Wnt/beta-catenin signaling regulates nephron induction during mouse kidney development. Development. 2007. 134:2533–2539.67. Georgas K, Rumballe B, Wilkinson L, Chiu HS, Lesieur E, Gilbert T, Little MH. Use of dual section mRNA in situ hybridisation/immunohistochemistry to clarify gene expression patterns during the early stages of nephron development in the embryo and in the mature nephron of the adult mouse kidney. Histochem Cell Biol. 2008. 130:927–942.68. Little MH, Brennan J, Georgas K, Davies JA, Davidson DR, Baldock RA, Beverdam A, Bertram JF, Capel B, Chiu HS, Clements D, Cullen-McEwen L, Fleming J, Gilbert T, Herzlinger D, Houghton D, Kaufman MH, Kleymenova E, Koopman PA, Lewis AG, McMahon AP, Mendelsohn CL, Mitchell EK, Rumballe BA, Sweeney DE, Valerius MT, Yamada G, Yang Y, Yu J. A high-resolution anatomical ontology of the developing murine genitourinary tract. Gene Expr Patterns. 2007. 7:680–699.69. Allanson JE, Hunter AG, Mettler GS, Jimenez C. Renal tubular dysgenesis: a not uncommon autosomal recessive syndrome: a review. Am J Med Genet. 1992. 43:811–814.70. Kopan R, Cheng HT, Surendran K. Molecular insights into segmentation along the proximal-distal axis of the nephron. J Am Soc Nephrol. 2007. 18:2014–2020.71. Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol. 2005. 288:F939–F952.72. McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, Herzlinger D, Weinmaster G, Jiang R, Gridley T. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001. 128:491–502.73. McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002. 129:1075–1082.74. Leheste JR, Melsen F, Wellner M, Jansen P, Schlichting U, Renner-Müller I, Andreassen TT, Wolf E, Bachmann S, Nykjaer A, Willnow TE. Hypocalcemia and osteopathy in mice with kidney-specific megalin gene defect. FASEB J. 2003. 17:247–249.75. Raila J, Willnow TE, Schweigert FJ. Megalin-mediated reuptake of retinol in the kidneys of mice is essential for vitamin A homeostasis. J Nutr. 2005. 135:2512–2516.76. Bachmann S, Schlichting U, Geist B, Mutig K, Petsch T, Bacic D, Wagner CA, Kaissling B, Biber J, Murer H, Willnow TE. Kidney-specific inactivation of the megalin gene impairs trafficking of renal inorganic sodium phosphate cotransporter (NaPi-IIa). J Am Soc Nephrol. 2004. 15:892–900.77. Bachmann S, Metzger R, Bunnemann B. Tamm-Horsfall protein-mRNA synthesis is localized to the thick ascending limb of Henle's loop in rat kidney. Histochemistry. 1990. 94:517–523.78. Raffi H, Bates JM, Laszik Z, Kumar S. Tamm-Horsfall protein knockout mice do not develop medullary cystic kidney disease. Kidney Int. 2006. 69:1914–1915.79. Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM, Bleyer AJ. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002. 39:882–892.80. Ge Y, Ahn D, Stricklett PK, Hughes AK, Yanagisawa M, Verbalis JG, Kohan DE. Collecting duct-specific knockout of endothelin-1 alters vasopressin regulation of urine osmolality. Am J Physiol Renal Physiol. 2005. 288:F912–F920.81. Rojek A, Füchtbauer EM, Kwon TH, Frøkiaer J, Nielsen S. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci U S A. 2006. 103:6037–6042.82. Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, Magnuson MA, Redha R, Zhang Y, Breyer MD. Thiazolidinediones expand body fluid volume through PPARgamma stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005. 11:861–866.83. Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003. 83:253–307.84. Tufro A, Norwood VF, Carey RM, Gomez RA. Vascular endothelial growth factor induces nephrogenesis and vasculogenesis. J Am Soc Nephrol. 1999. 10:2125–2134.85. Tufró A. VEGF spatially directs angiogenesis during metanephric development in vitro. Dev Biol. 2000. 227:558–566.86. Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003. 111:707–716.87. Lindahl P, Hellström M, Kalén M, Karlsson L, Pekny M, Pekna M, Soriano P, Betsholtz C. Paracrine PDGF-B/PDGF-Rbeta signaling controls mesangial cell development in kidney glomeruli. Development. 1998. 125:3313–3322.88. Ueda H, Miyazaki Y, Matsusaka T, Utsunomiya Y, Kawamura T, Hosoya T, Ichikawa I. Bmp in podocytes is essential for normal glomerular capillary formation. J Am Soc Nephrol. 2008. 19:685–694.89. Sadl V, Jin F, Yu J, Cui S, Holmyard D, Quaggin S, Barsh G, Cordes S. The mouse Kreisler (Krml1/MafB) segmentation gene is required for differentiation of glomerular visceral epithelial cells. Dev Biol. 2002. 249:16–29.90. Wharram BL, Goyal M, Gillespie PJ, Wiggins JE, Kershaw DB, Holzman LB, Dysko RC, Saunders TL, Samuelson LC, Wiggins RC. Altered podocyte structure in GLEPP1 (Ptpro)-deficient mice associated with hypertension and low glomerular filtration rate. J Clin Invest. 2000. 106:1281–1290.91. Koziell A, Grech V, Hussain S, Lee G, Lenkkeri U, Tryggvason K, Scambler P. Genotype/phenotype correlations of NPHS1 and NPHS2 mutations in nephrotic syndrome advocate a functional inter-relationship in glomerular filtration. Hum Mol Genet. 2002. 11:379–388.92. Kim JM, Wu H, Green G, Winkler CA, Kopp JB, Miner JH, Unanue ER, Shaw AS. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science. 2003. 300:1298–1300.93. Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996. 122:3537–3547.94. Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R. Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol. 2008. 316:288–301.95. Miner JH. Building the glomerulus: a matricentric view. J Am Soc Nephrol. 2005. 16:857–861.96. Longo I, Porcedda P, Mari F, Giachino D, Meloni I, Deplano C, Brusco A, Bosio M, Massella L, Lavoratti G, Roccatello D, Frascá G, Mazzucco G, Muda AO, Conti M, Fasciolo F, Arrondel C, Heidet L, Renieri A, De Marchi M. COL4A3/COL4A4 mutations: from familial hematuria to autosomal-dominant or recessive Alport syndrome. Kidney Int. 2002. 61:1947–1956.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetics of kidney development: pathogenesis of renal anomalies
- Renal Sodium Handling and Hypertension
- Ethical and Regulatory Problems of Molecular Imaging
- Molecular Regulation of Hypothalamic Development and Differentiation in Mammals
- Altered Renal Expression of Aquaporin-2 Water Channels in Two-Kidney, One Clip Hypertension in Rats