J Cardiovasc Ultrasound.
2014 Sep;22(3):127-133. 10.4250/jcu.2014.22.3.127.
The Value of Assessing Myocardial Deformation at Recovery after Dobutamine Stress Echocardiography
- Affiliations
-
- 1Department of Cardiology, Kyung Hee University Hospital at Gangdong, Kyung Hee University College of Medicine, Seoul, Korea. issohn@khu.ac.kr
- KMID: 2045428
- DOI: http://doi.org/10.4250/jcu.2014.22.3.127
Abstract
- BACKGROUND
The purpose of this study was to evaluate whether performing an assessment of myocardial deformation using speckle tracking imaging during the recovery period after dobutamine stress echocardiography (DSE) allows detection of significant coronary artery disease (CAD) in patients with chest discomfort.
METHODS
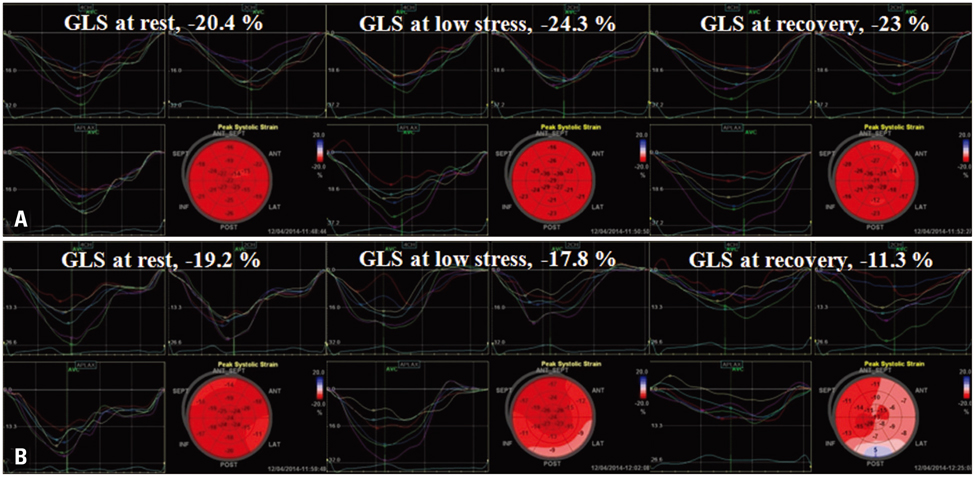
DSE and coronary angiography were performed in 44 patients with chest discomfort. The mean global longitudinal peak systolic strain (GLS) was measured at rest, at low stress (dobutamine infusion rate of 10 microg/kg/min) and at recovery (5 min after cessation of dobutamine infusion) of DSE using automated function imaging with apical views. Fractional flow reserve (FFR) was also performed in patients with intermediate coronary stenosis. CAD was defined as having a > or = 70% diameter stenosis on coronary angiography or as having a FFR < 0.8. Patients were divided two groups based on the absence or presence of CAD [CAD (-) group vs. CAD (+) group].
RESULTS
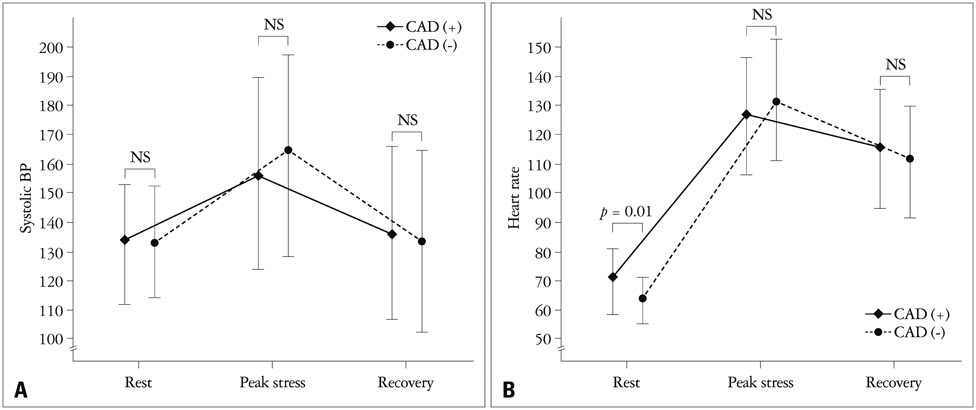
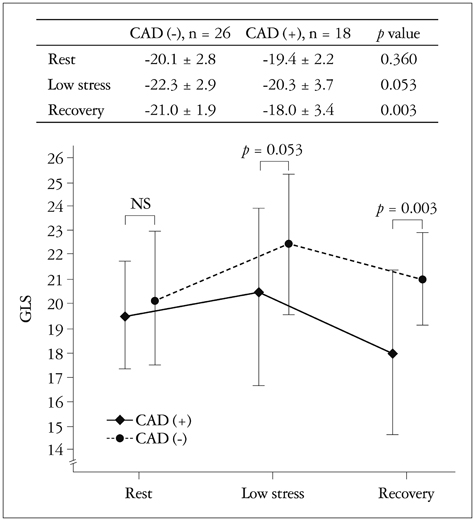
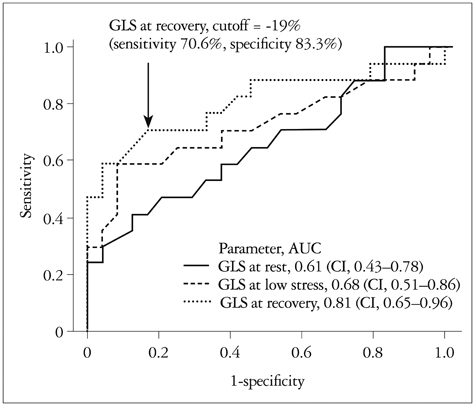
There were no significant differences in the clinical characteristics and results of conventional echocardiography between the two groups. GLS at recovery was lower in the CAD (+) group than in the CAD (-) group (-18.0 +/- 3.4% vs. -21.0 +/- 1.9%, p = 0.003). The optimal cutoff of GLS at recovery for detection of CAD was -19% (sensitivity of 70.6%, specificity of 83.3%).
CONCLUSION
Assessment of GLS at recovery of DSE is a reliable and objective method for detection of CAD. This finding may suggest that systolic myocardial stunning remains even after recovery of wall motion abnormalities in patients with CAD.
MeSH Terms
Figure
Reference
-
1. Szymanski C, Pierard L, Lancellotti P. Imaging techniques in coronary atherosclerotic disease: dobutamine stress echocardiography--evidence and perspectives. J Cardiovasc Med (Hagerstown). 2011; 12:543–553.2. Hoffmann R, Lethen H, Marwick T, Arnese M, Fioretti P, Pingitore A, Picano E, Buck T, Erbel R, Flachskampf FA, Hanrath P. Analysis of interinstitutional observer agreement in interpretation of dobutamine stress echocardiograms. J Am Coll Cardiol. 1996; 27:330–336.
Article3. Picano E, Lattanzi F, Orlandini A, Marini C, L'Abbate A. Stress echocardiography and the human factor: the importance of being expert. J Am Coll Cardiol. 1991; 17:666–669.
Article4. Anwar AM. Accuracy of two-dimensional speckle tracking echocardiography for the detection of significant coronary stenosis. J Cardiovasc Ultrasound. 2013; 21:177–182.
Article5. Bansal M, Jeffriess L, Leano R, Mundy J, Marwick TH. Assessment of myocardial viability at dobutamine echocardiography by deformation analysis using tissue velocity and speckle-tracking. JACC Cardiovasc Imaging. 2010; 3:121–131.
Article6. Voigt JU, Exner B, Schmiedehausen K, Huchzermeyer C, Reulbach U, Nixdorff U, Platsch G, Kuwert T, Daniel WG, Flachskampf FA. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation. 2003; 107:2120–2126.
Article7. Kloner RA, Bolli R, Marban E, Reinlib L, Braunwald E. Medical and cellular implications of stunning, hibernation, and preconditioning: an NHLBI workshop. Circulation. 1998; 97:1848–1867.
Article8. Tsoukas A, Ikonomidis I, Cokkinos P, Nihoyannopoulos P. Significance of persistent left ventricular dysfunction during recovery after dobutamine stress echocardiography. J Am Coll Cardiol. 1997; 30:621–626.
Article9. Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG. American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007; 20:1021–1041.
Article10. Nishimura RA, Miller FA Jr, Callahan MJ, Benassi RC, Seward JB, Tajik AJ. Doppler echocardiography: theory, instrumentation, technique, and application. Mayo Clin Proc. 1985; 60:321–343.
Article11. Tajik AJ, Seward JB, Hagler DJ, Mair DD, Lie JT. Two-dimensional real-time ultrasonic imaging of the heart and great vessels. Technique, image orientation, structure identification, and validation. Mayo Clin Proc. 1978; 53:271–303.12. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Chamber Quantification Writing Group. American Society of Echocardiography's Guidelines and Standards Committee. European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463.
Article13. Delgado V, Mollema SA, Ypenburg C, Tops LF, van der Wall EE, Schalij MJ, Bax JJ. Relation between global left ventricular longitudinal strain assessed with novel automated function imaging and biplane left ventricular ejection fraction in patients with coronary artery disease. J Am Soc Echocardiogr. 2008; 21:1244–1250.
Article14. De Bruyne B, Sarma J. Fractional flow reserve: a review: invasive imaging. Heart. 2008; 94:949–959.15. De Bruyne B, Pijls NH, Barbato E, Bartunek J, Bech JW, Wijns W, Heyndrickx GR. Intracoronary and intravenous adenosine 5'-triphosphate, adenosine, papaverine, and contrast medium to assess fractional flow reserve in humans. Circulation. 2003; 107:1877–1883.
Article16. Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996; 334:1703–1708.
Article17. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986; 1:307–310.
Article18. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845.
Article19. Ng AC, Sitges M, Pham PN, Tran da T, Delgado V, Bertini M, Nucifora G, Vidaic J, Allman C, Holman ER, Bax JJ, Leung DY. Incremental value of 2-dimensional speckle tracking strain imaging to wall motion analysis for detection of coronary artery disease in patients undergoing dobutamine stress echocardiography. Am Heart J. 2009; 158:836–844.
Article20. Hanekom L, Cho GY, Leano R, Jeffriess L, Marwick TH. Comparison of two-dimensional speckle and tissue Doppler strain measurement during dobutamine stress echocardiography: an angiographic correlation. Eur Heart J. 2007; 28:1765–1772.
Article21. Ishii K, Suyama T, Imai M, Maenaka M, Yamanaka A, Makino Y, Seino Y, Shimada K, Yoshikawa J. Abnormal regional left ventricular systolic and diastolic function in patients with coronary artery disease undergoing percutaneous coronary intervention: clinical significance of post-ischemic diastolic stunning. J Am Coll Cardiol. 2009; 54:1589–1597.
Article22. Kocabay G, Muraru D, Peluso D, Cucchini U, Mihaila S, Padayattil-Jose S, Gentian D, Iliceto S, Vinereanu D, Badano LP. Normal Left Ventricular Mechanics by Two-dimensional Speckle-tracking Echocardiography. Reference Values in Healthy Adults. Rev Esp Cardiol (Engl Ed). 2014; 67:651–658.
Article23. Dalen H, Thorstensen A, Romundstad PR, Aase SA, Stoylen A, Vatten LJ. Cardiovascular risk factors and systolic and diastolic cardiac function: a tissue Doppler and speckle tracking echocardiographic study. J Am Soc Echocardiogr. 2011; 24:322–332.e6.
Article24. Vinereanu D, Nicolaides E, Tweddel AC, Mädler CF, Holst B, Boden LE, Cinteza M, Rees AE, Fraser AG. Subclinical left ventricular dysfunction in asymptomatic patients with Type II diabetes mellitus, related to serum lipids and glycated haemoglobin. Clin Sci (Lond). 2003; 105:591–599.
Article25. Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, Voigt JU, Zamorano JL. European Association of Echocardiography. Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr. 2008; 9:415–437.
Article26. Ingul CB, Stoylen A, Slordahl SA, Wiseth R, Burgess M, Marwick TH. Automated analysis of myocardial deformation at dobutamine stress echocardiography: an angiographic validation. J Am Coll Cardiol. 2007; 49:1651–1659.27. Hoffmann R, Altiok E, Nowak B, Heussen N, Kühl H, Kaiser HJ, Büll U, Hanrath P. Strain rate measurement by doppler echocardiography allows improved assessment of myocardial viability inpatients with depressed left ventricular function. J Am Coll Cardiol. 2002; 39:443–449.
Article28. Gallagher KP, Osakada G, Matsuzaki M, Miller M, Kemper WS, Ross J Jr. Nonuniformity of inner and outer systolic wall thickening in conscious dogs. Am J Physiol. 1985; 249(2 Pt 2):H241–H248.
Article29. Zhang Q, Fung JW, Yip GW, Chan JY, Lee AP, Lam YY, Wu LW, Wu EB, Yu CM. Improvement of left ventricular myocardial short-axis, but not long-axis function or torsion after cardiac resynchronisation therapy: an assessment by two-dimensional speckle tracking. Heart. 2008; 94:1464–1471.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Low Dose Dobutamine Stress Echocardiography in Patients with Acute Myocardial Infarction
- The Usefulness of Dobutamine Stress Echocardiography for Evaluation of Viable Myocardium in Hibernating Myocardium
- Feasibility Study of Dobutamine Stress Transesophageal Echocardiography
- Comparison Study of Dipyridamole and Dobutamine Stress Echocardiography in Same Patients
- Significance of Left Ventricle Chamber Obliteration in Dobutamine Stress Echocardiography