J Korean Soc Radiol.
2010 May;62(5):411-419. 10.3348/jksr.2010.62.5.411.
Chronological Changes of the Signal Intensities of White Matter on the FLAIR Images of Infants
- Affiliations
-
- 1Department of Radiology, Dong-A University, College of Medicine, Busan, Korea. sschoi317@yahoo.co.kr
- KMID: 2002947
- DOI: http://doi.org/10.3348/jksr.2010.62.5.411
Abstract
- PURPOSE
To assess the pattern of chronological change for the signal intensities of white matter on the FLAIR images of infants.
MATERIALS AND METHODS
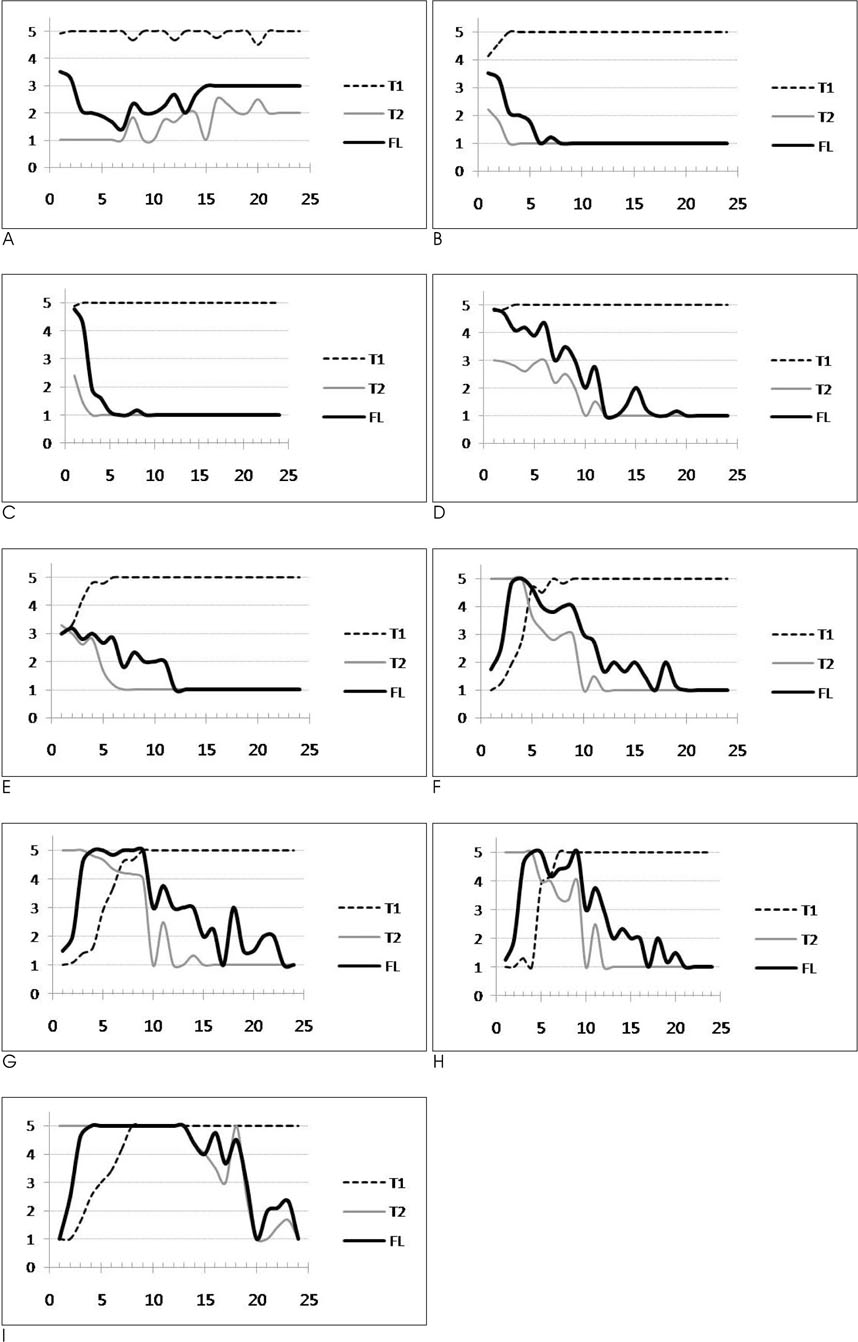
FLAIR, T1- and T2-weighted images of 119 infants (newborn to 24 months age) were obtained by using a 1.5 T MRI machine. From these images, the signal intensities of 9 different white matter regions were compared to those of the adjacent gray matter and the signal intensities of each image were scored from 1 to 5.
RESULTS
The FLAIR images show high signal intensity for the cerebellar peduncle and posterior limb of the internal capsule at birth, but changed to low signal intensities in 2 to 3 months. The low signal intensities of the occipital-, parietal-, and frontal deep white matter and subcortical white matter changed to high signal intensities in 2 months, and they returned to low signal intensities in 10, 11, 12 and 19 months, respectively.
CONCLUSION
The signal intensity of the white matter of infants showed peculiar chronologic changes on the FLAIR images. This knowledge could be helpful for the differentiation of normal white matter development and the lesions that mimic white matter disease in infants.
MeSH Terms
Figure
Reference
-
1. De Coene B, Hajnal JV, Gatehouse P, Longmore DB, White SJ, Oatridge A, et al. MR of the brain using fluid-attenuated inversion recovery (FLAIR) pulse sequences. AJNR Am J Neuroradiol. 1992; 13:1555–1564.2. Noguchi K, Ogawa T, Inugami A, Toyoshima H, Okudera T, Uemura K. MR of acute subarachnoid hemorrhage: a preliminary report of fluid-attenuated inversion-recovery pulse sequences. AJNR Am J Neuroradiol. 1994; 15:1940–1943.3. Ashikaga R, Araki Y, Ishida O. MRI of head injury using FLAIR. Neuroradiology. 1997; 39:239–242.4. Bakshi R, Kamran S, Kinkel PR, Bates VE, Mechtler LL, Janardhan V, et al. Fluid-attenuated inversion-recovery MR imaging in acute and subacute cerebral intraventricular hemorrhage. AJNR Am J Neuroradiol. 1999; 20:629–636.5. Okuda T, Korogi Y, Shigematsu Y, Sugahara T, Hirai T, Ikushima I, et al. Brain lesions: when should fluid-attenuated inversion-recovery sequences be used in MR evaluation? Radiology. 1999; 212:793–798.6. Dietrich RB, Bradley WG, Zaragoza EJ, Otto RJ, Taira RK, Wilson GH, et al. MR evaluation of early myelination patterns in normal and developmentally delayed infants. AJR Am J Roentgenol. 1988; 150:889–896.7. Barkovich AJ, Kjos BO, Jackson DE Jr, Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988; 166:173–180.8. Barkovich AJ, Lyon G, Evrard P. Formation, maturation, and disorders of white matter. AJNR Am J Neuroradiol. 1992; 13:447–461.9. Ballesteros MC, Hansen PE, Soila K. MR imaging of the developing human brain. Part 2. postnatal development. Radiographics. 1993; 13:611–622.10. Murakami JW, Weinberger E, Shaw DW. Normal myelination of the pediatric brain imaged with fluid-attenuated inversion recovery (FLAIR) MR imaging. AJNR Am J Neuroradiol. 1999; 20:1406–1411.11. Ashikaga R, Araki Y, Ono Y, Nishimura Y, Ishida O. Appearance of normal brain maturation on fluid-attenuated inversion recovery (FLAIR) MR images. AJNR Am J Neuroradiol. 1999; 20:427–431.12. Kizildağ B, Düşünceli E, Fitoz S, Erden I. The role of classic spin echo and FLAIR sequences for the evaluation of myelination in MR imaging. Diagn Interv Radiol. 2005; 11:130–136.13. Van der Knaap MS, Valk J. Magnetic Resonance of Myelin, Myelination and Myelin Disorders. 2nd ed. Berlin: Springer-Verlag;1995. p. 1–52.14. Dobbing J, Sands J. Quantitative growth and development of human brain. Arch Dis Child. 1973; 48:757–767.15. Hittmair K, Kramer J, Rand T, Bernert G, Wimberger D. Infratentorial brain maturation: a comparison of MRI at 0.5 and 1.5T. Neuroradiology. 1996; 38:360–366.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Study on CT Attenuation and MR Signal Intensity of Protein Solution
- Relative Signal Intensity Changes of Frontal and Occipital White Matters on T2 Weighted Axial MR Image: Correlation with Age
- Fast Fluid-Attenuated Inversion-Recovery MR Image in the Intracranial Tumors : Comparison with Fast Spin-echoImage
- Usefulness of Post-enhanced Delayed FLAIR Imaging for Making the Diagnosis of Leptomeningitis
- Comparison of Fast FLAIR and Echo-Planar FLAIR Imaging in Cere b ral Lesions