Korean J Urol.
2007 Apr;48(4):428-432. 10.4111/kju.2007.48.4.428.
Efficacy and Safety of Uro-Vaxom Treatment for Patients with Recurrent Cystitis: An Open Multicenter Study
- Affiliations
-
- 1Daniel Urology Clinic, Korea.
- 2Department of Urology, College of Medicine, The Catholic University of Korea, Seoul, Korea. cyh0831@catholic.ac.kr
- 3Department of Urology, College of Medicine, Hanyang University, Seoul, Korea.
- 4Department of Urology, College of Medicine, Kyungpook National University, Daegu, Korea.
- 5Department of Urology, College of Medicine, Kyung Hee University, Seoul, Korea.
- 6Department of Urology, College of Medicine, Soonchunhyang University, Bucheon, Korea.
- 7Department of Urology, College of Medicine, Chosun University, Gwangju, Korea.
- 8Department of Urology, College of Medicine, Korea University, Seoul, Korea.
- 9Department of Urology, College of Medicine, Ewha Womans University, Seoul, Korea.
- 10Department of Urology, College of Medicine, Chonbuk National University, Jeonju, Korea.
- 11Department of Urology, College of Medicine, Yonsei University, Seoul, Korea.
- 12Department of Urology, College of Medicine, Inje University, Korea.
- 13Department of Urology, College of Medicine, Pusan National University, Busan, Korea.
- KMID: 1997111
- DOI: http://doi.org/10.4111/kju.2007.48.4.428
Abstract
- PURPOSE
We wanted to investigate the efficacy and safety of the immunotherapeutic Uro-Vaxom for treating uncomplicated recurrent cystitis in female patients only.
MATERIALS AND METHODS
Adult female patients were enrolled in this multicenter, open-label study if they had acute cystitis at the enrollment visit and positive results on urine culture (> or =10(3)CFU/ml). The patients were treated for 3 months with one capsule daily of Uro-Vaxom after antibiotic therapy, and they were observed for another 3 months. The primary efficacy criteria were the cystitis recurrence rates over 6 months, the distribution of cystitis and the proportion of patients with cystitis.
RESULTS
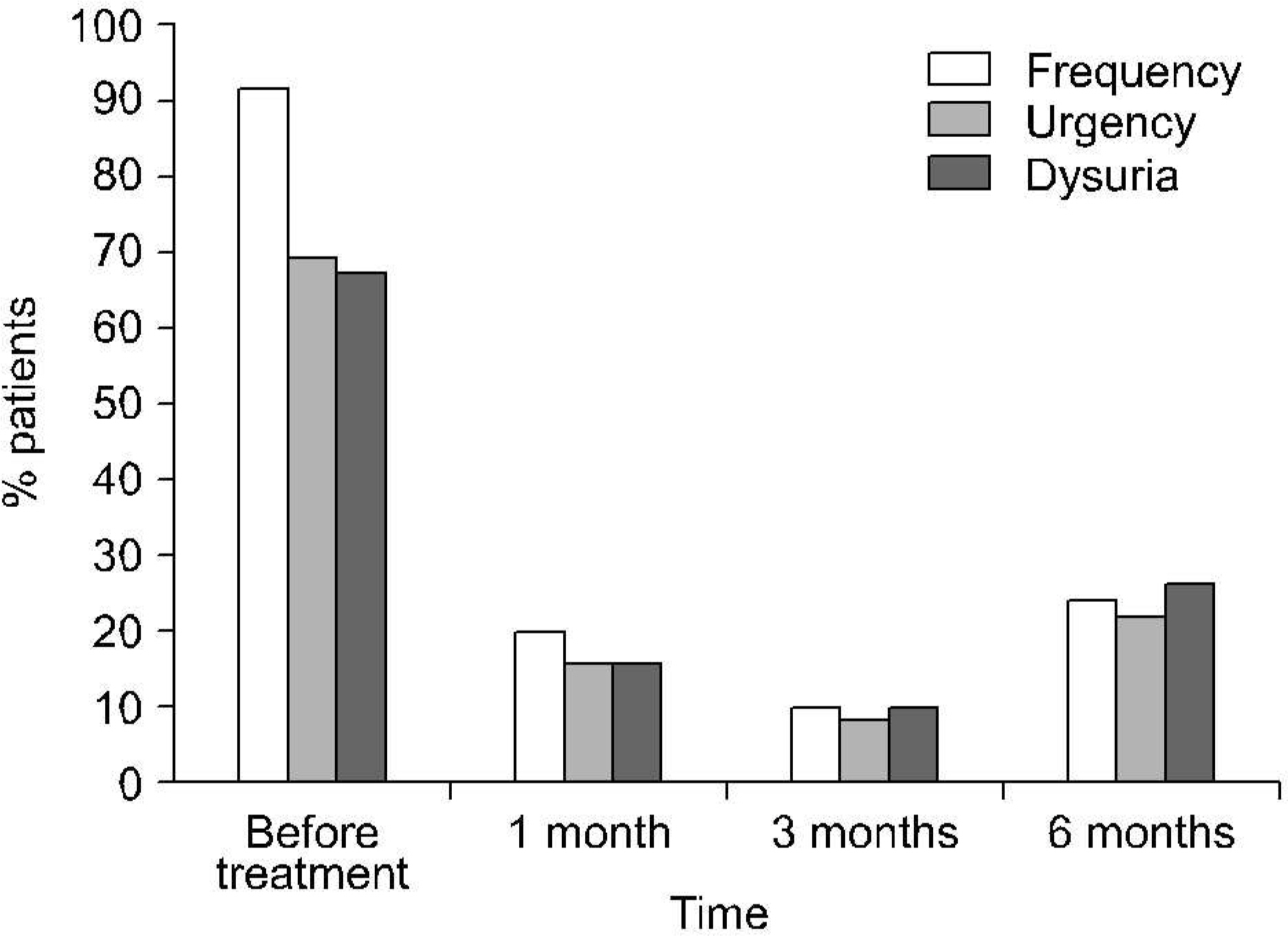
A total of 50 patients were evaluated. During the 6-month trial, the number of cystitis recurrences was significantly reduced in comparison with the 6-month pretrial period (on the average 0.64 as compared to 3.0 recurrences, respectively p<0.001). The incidences of frequency, urgency and dysuria remained low until the end of the trial. Uro-Vaxom was well tolerated: side-effects were mentioned by 8% of the 50 patients, and there was no case leading to treatment withdrawal.
CONCLUSIONS
Uro-Vaxom significantly reduced the incidence of cystitis during the 6 months of this study, including the 3 months of treatment. These results demonstrate that Uro-Vaxom is a valuable agent for prophylaxis of recurrent cystitis.
Keyword
Figure
Cited by 3 articles
-
A Prospective Multi-center Trial of Escherichia coli Extract for the Prophylactic Treatment of Patients with Chronically Recurrent Cystitis
Kun Suk Kim, Ji-Yoon Kim, In Gab Jeong, Jae-Seung Paick, Hwancheol Son, Dae Jung Lim, Hong Bang Shim, Won Hee Park, Hee Chang Jung, Myung-Soo Choo
J Korean Med Sci. 2010;25(3):435-439. doi: 10.3346/jkms.2010.25.3.435.Non-Antibiotic Prophylaxis for Recurrent Urinary Tract Infections
Ki Ho Kim
Korean J Urogenit Tract Infect Inflamm. 2014;9(1):9-13. doi: 10.14777/kjutii.2014.9.1.9.Risk Factor Associated with Recurrence after OM-89 (Uro-Vaxom®) Treatment for Female Recurrent Cystitis
Ji-Yeon Han
Urogenit Tract Infect. 2019;14(2):42-45. doi: 10.14777/uti.2019.14.2.42.
Reference
-
1.Sobel JD. Bacterial etiologic agents in the pathogenesis of urinary tract infection. Med Clin North Am. 1991. 75:253–73.
Article2.Valiquette L. Urinary tract infections in women. Can J Urol. 2001. 8(Suppl 1):6–12.3.Foxman B., Barlow R., D' Arcy H., Gillespie B., Sobel JD. Urinary tract infecion: self-reported incidence and associated costs. Ann Epidemiol. 2000. 10:509–15.4.Pfau A., Sacks TG. Effective postcoital quinolone prophylaxis of recurrent urinary tract infections in women. J Urol. 1994. 152:136–8.
Article5.Kahlmeter GJ. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project. J Antimicrob Chemother. 2003. 51:69–76.6.Karlowsky JA., Jones ME., Thonsberrry C., FriedlaM IR., Sahm DF. Trends in antimicrobial susceptibilities among enterobacteriaceae isolated from hospitalized patients in the United States from 1998 to 2001. Antimicrob Agents Chemodier. 2003. 47:1672–80.7.Krieger JN. Urinary tract infections: what' s new? J Urol. 2002. 168:2351–8.8.Olson NC., Hellyer PW., Dodam JR. Mediators and vascular effects in response to endotoxin. Br Vet J. 1995. 151:489–522.9.Hockertz S. Immunomodulating effect of lysed immunoactive fractions of selected Escherichia coli strains on the macrophage system. An in vitro study. Arzneimittelforschung. 1990. 40:1068–72.10.Lee SJ., Kim SW., Cho YH., Yoon MS. Anti-inflammatory effect of an Escherichia coli extract in a mouse model of lipopolysaccharide-induced cystitis. World J Urol. 2006. 24:33–8.
Article11.Bessler WG., Kleine B., Martinez Alonso C., Biesert L., Strecker M., Wiesmuller KH, et al. Biological activity of bacterial surface components: bacterial extracts and defined bacterial cell wall components as immunomodulators. Lung. 1990. 168((Suppl):):707–15.
Article12.Bessler WG., Beck P., Konetznick U., Loleit M., Sedelmeier E., Hoffmann P, et al. Biological activity of bacterial surface components. Immunogenicity and immunomodulatory properties of a bacterial extract from Escherichia coli. Arzneimittelforschung. 1991. 41:274–9.13.Hachen HJ. Oral immunotherapy in paraplegic patients with chronic urinary tract infections: a double-blind, placebocontrolled trial. J Urol. 1990. 143:759–62.
Article14.Schulman CC., Corbusier A., Michiels H., Taenzer HJ. Oral immunotherapy of recurrent urinary tract infections: a doubleblind placebo-controlled multicenter study. J Urol. 1993. 150:917–21.
Article15.Magasi P., Panovics J., Hies A., Nagy M. Uro-Vaxom and the management of recurrent urinary tract infection in adults: a randomized multicenter double-blind trial. Eur Urol. 1994. 26:137–40.16.Lettgen B. Prevention of recurrent urinary tract infections in female children. Curr Ther Res. 1996. 57:464–75.
Article17.Wybran J., Libin M., Schandene L. Enhancement of cytokine production and natural killer activity by an Escherichia coli extract. Onkologie. 1989. 12(Suppl 3):22–5.
Article18.Korean Continence Society. Textbook of voiding dysfunction and female urology. 1st ed.Seoul: Ilchokak Publishing Inc;2003. p. 102–3.19.Stamm WE., McKevitt M., Roberts PL., White NJ. Natural history of recurrent urinary infections in women. Rev Infect Dis. 1991. 13:77–84.20.Kass EH. Asymptomatic infections of die urinary tract. Trans Assoc Am Physicians. 1956. 69:56–64.21.Stamm WE., Counts GW., Running KR., Fihn S., Turck M., Holmes KK. Diagnosis of coliform infection in acutely dysuric women. N Engl J Med. 1982. 307:463–8.
Article22.Rubin RH., Shapiro ED., Andriole VT., Davis RJ., Stamm WE. Evaluation of new anti-infective drugs for the treatment of urinary tract infection. Infectious Diseases Society of America and die Food and Drug Administration. Clin Infect Dis. 1992. 15(Suppl l):216–27.23.Rubin RH., Shapiro ED., Andriole VT., Davis RJ., Stamm WE. General guidelines for die evaluation of new anti-infective drugs for the treatment of UTI. Taufkirchen, Germany: The European Society of Clinical Microbiology and Infectious Diseases;1993. p. 240–310.24.Bauer HW., Alloussi S., Egger G., Blumlein HM., Cozma G., Schulman CC, et al. A long-term, multicenter, double-blind study of an Escherichia coli extract (OM-89) in female patients with recurrent urinary tract infections. Eur Urol. 2005. 47:542–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Clinical Efficacy of Uro-Vaxom(R)in the Treatment of Recurrent Urinary Tract Infections
- The Recurrence of Chronic Pelvic Pain Syndrome and the Role of Uro-Vaxom(R)
- The Application of Uro-vaxom(R), Urinary Tract Immunostimulator in the Treatment of Chronic Pelvic Pain Syndrome
- A Prospective Multi-center Trial of Escherichia coli Extract for the Prophylactic Treatment of Patients with Chronically Recurrent Cystitis
- Importance to Promote Awareness in Patients with Recurrent Cystitis