Korean J Urol.
2010 Aug;51(8):572-578. 10.4111/kju.2010.51.8.572.
Direct Effect of Carbon Monoxide on Relaxation Induced by Electrical Field Stimulation in Rat Corpus Cavernosum
- Affiliations
-
- 1Department of Urology, Chonbuk National University Medical School, Jeonju, Korea. rain@chonbuk.ac.kr
- 2Institute for Medical Sciences of Chonbuk National University, Jeonju, Korea.
- 3Research Institute of Clinical Medicine, Chonbuk National University Hospital, Jeonju, Korea.
- 4CTC for Medical Device of Chonbuk National University Hospital, Jeonju, Korea.
- KMID: 1997027
- DOI: http://doi.org/10.4111/kju.2010.51.8.572
Abstract
- PURPOSE
Carbon monoxide (CO) may mediate smooth muscle relaxation in the rat corpus cavernosum smooth muscle (CCSM). We hypothesized that CO plays a role in neurally derived, frequency-dependent relaxation of rat CCSM.
MATERIALS AND METHODS
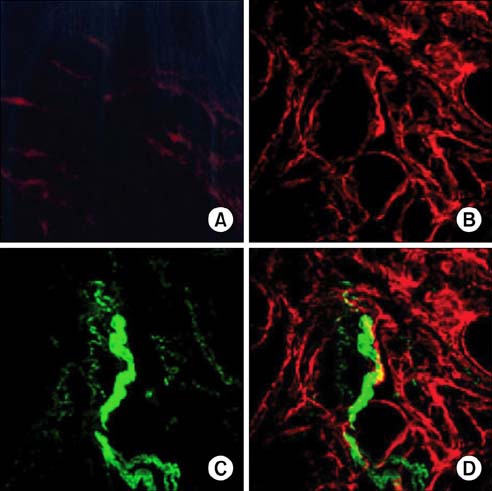
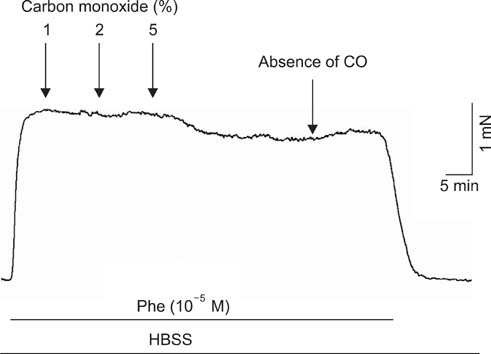
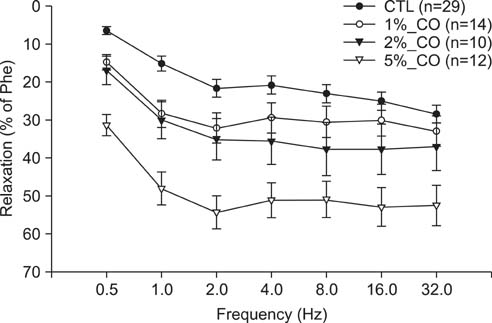
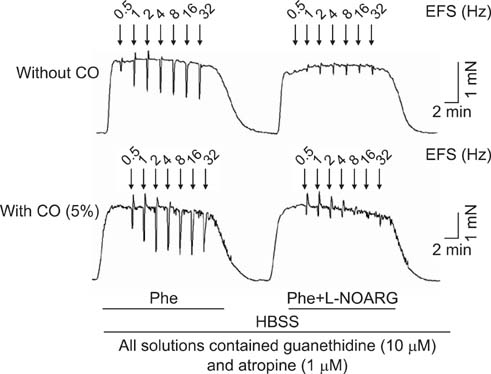
To study the effect of CO on CCSM relaxation induced by electrical field stimulation (EFS), a CCSM bundle was mounted on a force transducer and perfused with Hanks' balanced salt solution at 37degrees C with 95% O2 and 5% CO2. After 1 hour equilibration with -500 mg of passive tension, contraction of the CCSM bundle was elicited by 10(-5) M phenylephrine, which was continuously added with different concentrations of CO (1%, 2%, and 5%). Frequency-dependent relaxation was induced by EFS trains (0.2 ms at 0.5-32 Hz, for 10 s) repeated at 2 min intervals over 15 min in the presence of adrenergic and muscarinic receptor blocking agents (guanethidine and atropine, respectively). To study the distribution of heme oxygenase-2 (HO-2) in the rat CCSM, we performed immunohistochemical evaluation.
RESULTS
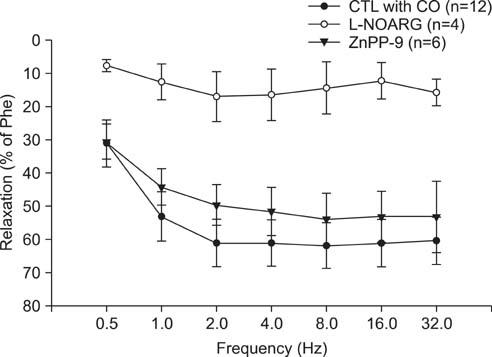
CO produced a dose-dependent enhancement of EFS-induced relaxation. Pretreatment with N(G)-nitro-L-arginine (a nitric oxide synthase blocker) greatly reduced the EFS-induced relaxation in the presence of CO (-45%). Pretreatment with zinc protoporphyrin-IX (ZnPP-9, a heme oxygenase inhibitor) had no significant effect on EFS-induced relaxation in the absence or the presence of CO. We found immunoreactivity for HO-2 in CCSM and immunoreactivity for protein gene product 9.5 (PGP 9.5) in nerve fibers.
CONCLUSIONS
We conclude that CO produced a dose-dependent enhancement of EFS-induced relaxation in rat CCSM bundles, but neurally derived, frequency-dependent relaxation in the rat CCSM depended mostly on nitric oxide in response to nonadrenergic noncholinergic neurotransmission. Immunoreactivity for HO-2 was found in rat CCSM but not nerve fibers.
MeSH Terms
-
Animals
Atropine
Carbon
Carbon Monoxide
Contracts
Cyclic GMP
Electric Stimulation
Heme
Heme Oxygenase (Decyclizing)
Isotonic Solutions
Male
Muscle Relaxation
Muscle, Smooth
Nerve Fibers
Nitric Oxide
Nitric Oxide Synthase
Penile Erection
Phenylephrine
Rats
Receptors, Muscarinic
Relaxation
Synaptic Transmission
Transducers
Zinc
Atropine
Carbon
Carbon Monoxide
Cyclic GMP
Heme
Heme Oxygenase (Decyclizing)
Isotonic Solutions
Nitric Oxide
Nitric Oxide Synthase
Phenylephrine
Receptors, Muscarinic
Zinc
Figure
Reference
-
1. Gross SS, Wolin MS. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995. 57:737–769.2. Andersson KE, Stief CG. Neurotransmission and the contraction and relaxation of penile erectile tissue. World J Urol. 1997. 15:14–20.3. Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995. 57:707–736.4. Umans JG, Levi R. Nitric oxide in the regulation of blood flow and arterial pressure. Annu Rev Physiol. 1995. 57:771–790.5. Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988. 2:2557–2568.6. Bredt DS, Hwang PM, Glatt CE, Lowenstein C, Reed RR, Snyder SH. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991. 351:714–718.7. Marks GS, Brien JF, Nakatsu K, McLaughlin BE. Does carbon monoxide have a physiologic function? Trends Pharmacol Sci. 1991. 12:185–188.8. Gräser T, Vedernikov YP, Li DS. Study on the mechanism of carbon monoxide induced endothelium-independent relaxation in porcine coronary artery and vein. Biomed Biochim Acta. 1990. 49:293–296.9. Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991. 28:52–61.10. Brian JE Jr, Heistad DD, Faraci FM. Effect of carbon monoxide on rabbit cerebral arteries. Stroke. 1994. 25:639–643.11. Kim YC, Davies MG, Marson L, Hagen PO, Carson CC 3rd. Lack of effect of carbon monoxide inhibitor on relaxation induced by electrical field stimulation in corpus cavernosum. Urol Res. 1994. 22:291–293.12. Ignarro LJ, Bush PA, Buga GM, Wood KS, Fukuto JM, Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990. 170:843–850.13. Xue L, Farrugia G, Miller SM, Ferris CD, Snyder SH, Szurszewski JH. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc Natl Acad Sci USA. 2000. 97:1851–1855.14. Rattan S, Chakder S. Inhibitory effect of CO on internal anal sphincter: heme oxygenase inhibitor inhibits NANC relaxation. Am J Physiol. 1993. 265:G799–G804.15. McCoubrey WK Jr, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997. 247:725–732.16. Grozdanovic Z, Gossrau R. Expression of heme oxygenase-2 (HO-2)-like immunoreactivity in rat tissues. Acta Histochem. 1996. 98:203–214.17. Ingi T, Cheng J, Ronnett GV. Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system. Neuron. 1996. 16:835–842.18. Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol. 1999. 277:F882–F889.19. Morse D, Sethi J, Choi AM. Carbon monoxide-dependent signaling. Crit Care Med. 2002. 30:1 Suppl. S12–S17.20. Zakhary R, Gaine SP, Dinerman JL, Ruat M, Flavahan NA, Snyder SH. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc Natl Acad Sci USA. 1996. 93:795–798.21. Vedernikov YP, Gräser T, Vanin AF. Similar endothelium-independent arterial relaxation by carbon monoxide and nitric oxide. Biomed Biochim Acta. 1989. 48:601–603.22. Wu L, Cao K, Lu Y, Wang R. Different mechanisms underlying the stimulation of KCa channels by nitric oxide and carbon monoxide. J Clin Invest. 2002. 110:691–700.23. Tamura M, Kagawa S, Kimura K, Kawanishi Y, Tsuruo Y, Ishimura K. Coexistence of nitric oxide synthase, tyrosine hydroxylase and vasoactive intestinal polypeptide in human penile tissue--a triple histochemical and immunohistochemical study. J Urol. 1995. 153:530–534.24. White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br J Pharmacol. 1997. 122:1573–1584.25. Ewing JF, Raju VS, Maines MD. Induction of heart heme oxygenase-1 (HSP32) by hyperthermia: possible role in stress-mediated elevation of cyclic 3':5'-guanosine monophosphate. J Pharmacol Exp Ther. 1994. 271:408–414.26. McMillan K, Bredt DS, Hirsch DJ, Snyder SH, Clark JE, Masters BS. Cloned, expressed rat cerebellar nitric oxide synthase contains stoichiometric amounts of heme, which binds carbon monoxide. Proc Natl Acad Sci USA. 1992. 89:11141–11145.27. Vincent SR, Das S, Maines MD. Brain heme oxygenase isoenzymes and nitric oxide synthase are co-localized in select neurons. Neuroscience. 1994. 63:223–231.28. Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res. 1997. 80:557–564.29. Dwyer BE, Nishimura RN, Lu SY. Differential localization of heme oxygenase and NADPH-diaphorase in spinal cord neurons. Neuroreport. 1995. 6:973–976.30. Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated bt smooth muscle cell-derived carbon monoxide. J Clin Invest. 1995. 96:2676–2682.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Electrical Field Stimulation of the Isolated Corpus Cavernosum from Hypertensive Rats
- Local Effect of Psychotherapeutic Agents on Rabbit Penile Corpus Cavernosum
- An Experimental Study of the Effect of Histamine on the Human Corpus Cavernosum Tissue
- Pharmacologic Effect of Phentolamine on Norepinephrine Induced Contraction of Corpus Cavernosum
- An Experimental Study of the Effect of Histamine on the Rabbit Corpus Cavernosum Tissue