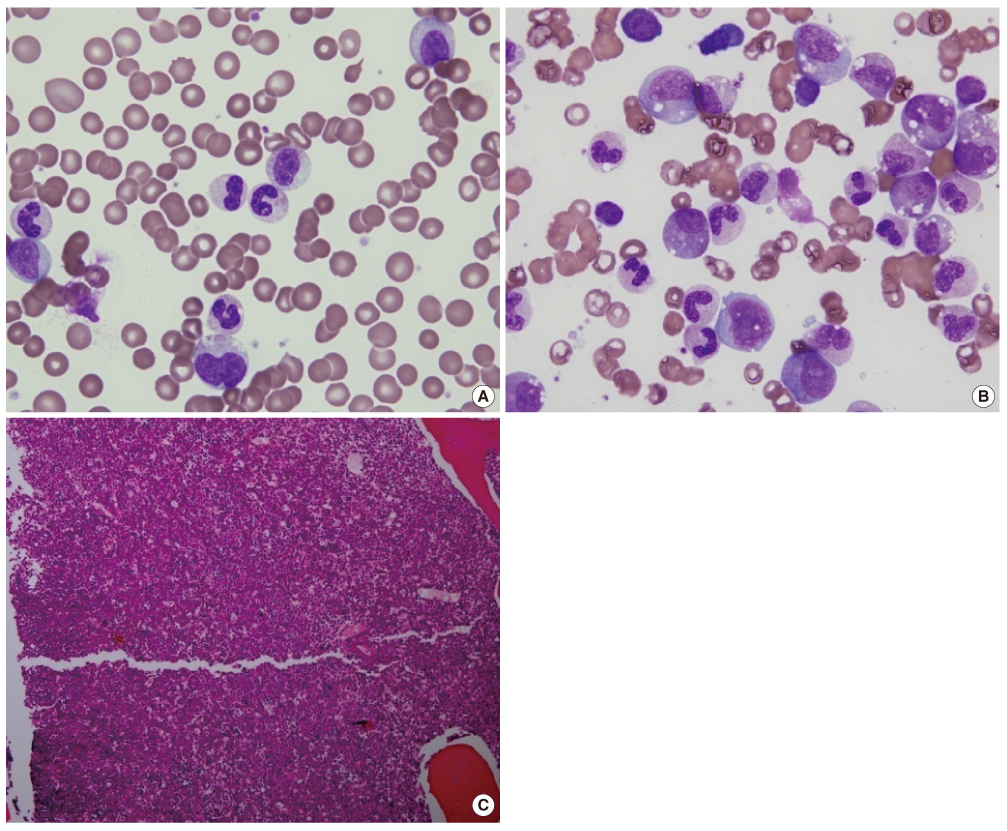
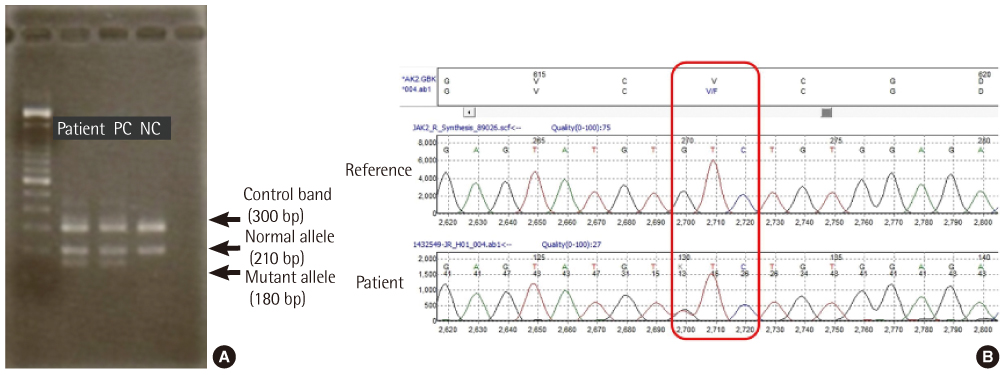
Lab Med Online.
2011 Oct;1(4):232-236. 10.3343/lmo.2011.1.4.10.
A Case of Atypical Chronic Myeloid Leukemia with the JAK2V617F Mutation
- Affiliations
-
- 1Department of Laboratory Medicine, Korea University College of Medicine, Seoul, Korea. yuret@korea.ac.kr
- 2Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea.
- KMID: 1993642
- DOI: http://doi.org/10.3343/lmo.2011.1.4.10
Abstract
- Atypical chronic myeloid leukemia (aCML) is a rare leukemic disorder that shows myelodysplastic and myeloproliferative features simultaneously. The Janus kinase 2 gene V617F mutation (JAK2V617F) in aCML has been the source of much controversy. Some JAK2V617F positive cases have been reported but others observed no JAK2V617F mutation in aCML as defined by WHO classification. Recently, we experienced a case of aCML with JAK2V617F mutation with typical myelodysplastic/myeloproliferative features in peripheral blood and bone marrow aspirates. The karyotype was normal and no BCR/ABL1, PDGFRA or PDGFRB gene rearrangement was noted with FISH analysis. JAK2V617F mutation of the case was identified with amplification refractory mutation system PCR and direct sequencing. We also studied JAK2V617F mutation status in 3 additional cases of previously diagnosed aCML in our institution, but no mutation was identified.
Keyword
MeSH Terms
Figure
Reference
-
1. Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick H, et al. Proposals by the French-American-British Cooperative Leukaemia Group. The chronic myeloid leukaemias: guidelines for distinguishing chronic granulocytic, atypical chronic myeloid, and chronic myelomonocytic leukaemia. Br J Haematol. 1994. 87:746–754.
Article2. Swerdlow SH, Campo E, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 2008. 4th ed. Lyon, France: IARC Press;80–81.3. Hall J, Foucar K. Diagnosing myelodysplastic/myeloproliferative neoplasms: laboratory testing strategies to exclude other disorders. Int J Lab Hematol. 2010. 32:559–571.
Article4. Fend F, Horn T, Koch I, Vela T, Orazi A. Atypical chronic myeloid leukemia as defined in the WHO classification is a JAK2 V617F negative neoplasm. Leuk Res. 2008. 32:1931–1935.
Article5. Campiotti L, Uccella S, Appio L, Pallotti F, La Rosa S, Capella C, et al. JAK2 mutation and atypical chronic myeloid leukemia. Leuk Res. 2009. 33:e166–e167.
Article6. Yoo SJ, Kang SG, Seo EJ, Park CJ, Lee KH, Chi HS. Four cases of atypical chronic myeloid leukemia. Korean J Clin Pathol. 2002. 22:75–79.7. Kim HW, Lee SS, Ryu MH, Lee JL, Chang HM, Kim TW, et al. A case of leukemic pleural infiltration in atypical chronic myeloid leukemia. J Korean Med Sci. 2006. 21:936–939.
Article8. Cho HS, Lee CH, Kim KD, Lee KH, Bae SH, Cho D, et al. A case of myeloid blast crisis of atypical chronic myelogenous leukemia. Korean J Hematol. 2003. 38:200–204.9. Jones AV, Kreil S, Zoi K, Waghorn K, Curtis C, Zhang L, et al. Widespread occurrence of the JAK2 V617F mutation in chronic myeloproliferative disorders. Blood. 2005. 106:2162–2168.
Article10. Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M, Stoffregen E, et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005. 106:3377–3379.
Article11. Jelinek J, Oki Y, Gharibyan V, Bueso-Ramos C, Prchal JT, Verstovsek S, et al. JAK2 mutation 1849G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood. 2005. 106:3370–3373.
Article12. Boyle EB, Steinbuch M, Tekautz T, Gutman JR, Robison LL, Perentesis JP. Accuracy of DNA amplification from archival hematological slides for use in genetic biomarker studies. Cancer Epidemiol Biomarkers Prev. 1998. 7:1127–1131.13. Ernst T, Chase A, Zoi K, Waghorn K, Hidalgo-Curtis C, Score J, et al. Transcription factor mutations in myelodysplastic/myeloproliferative neoplasms. Haematologica. 2010. 95:1473–1480.
Article14. Pérez B, Kosmider O, Cassinat B, Renneville A, Lachenaud J, Kaltenbach S, et al. Genetic typing of CBL, ASXL1, RUNX1, TET2 and JAK2 in juvenile myelomonocytic leukaemia reveals a genetic profile distinct from chronic myelomonocytic leukaemia. Br J Haematol. 2010. 151:460–468.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Leukemia Cutis in Myelodysplastic Syndrome Evolving into An Atypical Chronic Myeloid Leukemia
- Four Cases of Atypical Chronic Myeloid Leukemia
- A Pediatric Case of Atypical Chronic Myeloid Leukemia with CSF3R Mutation Not Responding to Ruxolitinib, but Rescued by Allogeneic Transplantation
- Acute myeloid leukemia arising from chronic myelomonocytic leukemia during hypomethylating therapy
- A Case of Myeloid Blast Crisis of Atypical Chronic Myelogenous Leukemia