Analysis of Immunoglobulin Gene Rearrangement: Comparison between BIOMED-2 Multiplex PCR and Conventional Nested PCR
- Affiliations
-
- 1Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. yhko310@skku.edu
- 2Department of Laboratory Medicine & Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Pathology, Dankook University Hospital, Dankook University School of Medicine, Cheonan, Korea.
- KMID: 1993637
- DOI: http://doi.org/10.3343/lmo.2011.1.4.5
Abstract
- BACKGROUND
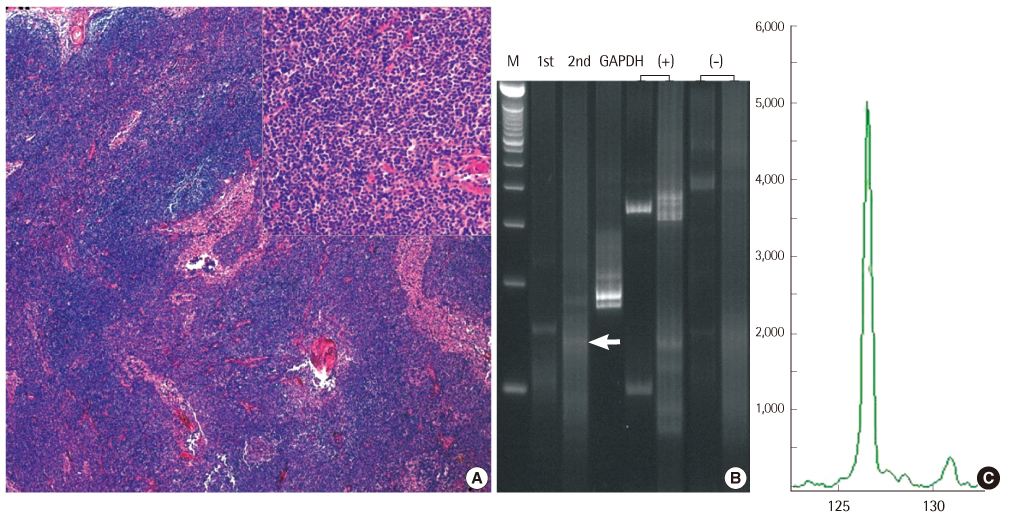
Immunoglobulin (Ig) gene rearrangement analysis is a useful additional tool to detect clonality of B-lymphoproliferative disease and the method to detect immunoglobulin gene rearrangement is required the high sensitivity and specificity. BIOMED-2 multiplex PCR was designed for the evaluation of molecular clonality of lymphoid lesions. We evaluated the usefulness of the BIOMED-2 multiplex PCR by comparing it with conventional nested PCR.
METHODS
Sixteen patients with malignant lymphoma and 5 with reactive lymph nodes were examined to assess the sensitivity, specificity, and accuracy between conventional nested PCR and BIOMED-2. All 3 tests performed using the BIOMED-2 kit for immunoglobulin (Ig) heavy chain (IGH) gene, Igkappa light chain (IGK) gene, and Iglambda light chain (IGL) gene, were used to analyze clonality.
RESULTS
Both the methods showed 100% specificity (95% confidence interval, 56.6-100.0). The combination of IGH and IGK BIOMED-2 tests with or without IGL revealed the highest sensitivity (87.5%; range, 64.0-96.5%) and accuracy (90%; range, 0.70-0.97). Compared to the conventional method, the BIOMED-2 test for IGH showed a higher sensitivity (62.5%; range, 38.6-81.5%) and accuracy (71%, 0.50-0.86).
CONCLUSIONS
These results suggest that, compared to the conventional method, BIOMED-2 has higher sensitivity and allows for easier interpretation while evaluating the clonality of B-lymphoproliferative disease.
MeSH Terms
Figure
Cited by 2 articles
-
Clinical Significance of Clonal Rearrangement of the Immunoglobulin Gene in the Bone Marrow of Patients with B-cell Non-Hodgkin Lymphoma
Ji Hyun Kim, Ja Young Lee, Jong Ae Son, Sae Am Song, Seung Hwan Oh, Jeong Hwan Shin, Hye Ran Kim, Kyung Ran Jun, Jeong Nyeo Lee
Lab Med Online. 2014;4(3):125-131. doi: 10.3343/lmo.2014.4.3.125.Diagnostic Accuracy and Prognostic Relevance of Immunoglobulin Heavy Chain Rearrangement and 18F-FDG-PET/CT Compared With Unilateral Bone Marrow Trephination for Detecting Bone Marrow Involvement in Patients With Diffuse Large B-Cell Lymphoma
Mihee Kim, Seo-Yeon Ahn, Jae-Sook Ahn, Ga-Young Song, Sung-Hoon Jung, Je-Jung Lee, Hyeoung-Joon Kim, Jun Hyung Lee, Myung-Geun Shin, Sang Yun Song, Deok-Hwan Yang
J Korean Med Sci. 2021;37(1):e2. doi: 10.3346/jkms.2022.37.e2.
Reference
-
1. Roman E, Smith AG. Epidemiology of lymphomas. Histopathology. 2011. 58:4–14.
Article2. Cook JR. Tubbs RR, Stoler MH, editors. Molecular Hematopathology. Cell and tissue based molecular pathology: a volume in the series foundations in diagnostic pathology. 2008. 1st ed. Philadelphia, PA: Churchill Livingstone;305–324.
Article3. Davis TH, Yockey CE, Balk SP. Detection of clonal immunoglobulin gene rearrangements by polymerase chain reaction amplification and single-strand conformational polymorphism analysis. Am J Pathol. 1993. 142:1841–1847.4. Linke B, Bolz I, Fayyazi A, von Hofen M, Pott C, Bertram J, et al. Automated high resolution PCR fragment analysis for identification of clonally rearranged immunoglobulin heavy chain genes. Leukemia. 1997. 11:1055–1062.
Article5. van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 concerted action BMH4-CT98-3936. Leukemia. 2003. 17:2257–2317.
Article6. Burack WR, Laughlin TS, Friedberg JW, Spence JM, Rothberg PG. PCR assays detect B-lymphocyte clonality in formalin-fixed, paraffin-embedded specimens of classical hodgkin lymphoma without microdissection. Am J Clin Pathol. 2010. 134:104–111.
Article7. Evans PA, Pott Ch, Groenen PJ, Salles G, Davi F, Berger F, et al. Significantly improved PCR-based clonality testing in B-cell malignancies by use of multiple immunoglobulin gene targets. Report of the BIOMED-2 concerted action BHM4-CT98-3936. Leukemia. 2007. 21:207–214.
Article8. Hebeda KM, Van Altena MC, Rombout P, van Krieken JH, Groenen PJ. PCR clonality detection in Hodgkin lymphoma. J Hematop. 2009. 2:34–41.
Article9. Oh HR, Lee MJ, Park G, Moon DS, Park YJ, Jang SJ. A case of lambda-expressing pulmonary MALT lymphoma with dual clonal rearrangements of kappa and lambda immunoglobulin light chain gene. Korean J Lab Med. 2009. 29:256–261.
Article10. Achille A, Scarpa A, Montresor M, Scardoni M, Zamboni G, Chilosi M, et al. Routine application of polymerase chain reaction in the diagnosis of monoclonality of B-cell lymphoid proliferations. Diagn Mol Pathol. 1995. 4:14–24.
Article11. Chute DJ, Cousar JB, Mahadevan MS, Siegrist KA, Silverman LM, Stoler MH. Detection of immunoglobulin heavy chain gene rearrangements in classic hodgkin lymphoma using commercially available BIOMED-2 primers. Diagn Mol Pathol. 2008. 17:65–72.
Article12. Male D, Brostoff J, editors. Immunology. 2006. 7th ed. Milton Keynes, United Kingdom: Mosby;80–85.13. Bagg A, Braziel RM, Arber DA, Bijwaard KE, Chu AY. Immunoglobulin heavy chain gene analysis in lymphomas: a multi-center study demonstrating the heterogeneity of performance of polymerase chain reaction assays. J Mol Diagn. 2002. 4:81–89.14. Tümkaya T, Beishuizen A, Wolvers-Tettero IL, van Dongen JJ. Identification of immunoglobulin lambda isotype gene rearrangements by Southern blot analysis. Leukemia. 1996. 10:1834–1839.15. van der Burg M, Tümkaya T, Boerma M, de Bruin-Versteeg S, Langerak AW, van Dongen JJ. Ordered recombination of immunoglobulin light chain genes occurs at the IGK locus but seems less strict at the IGL locus. Blood. 2001. 97:1001–1008.
Article16. van Krieken JH, Langerak AW, Macintyre EA, Kneba M, Hodges E, Sanz RG, et al. Improved reliability of lymphoma diagnostics via PCR-based clonality testing: report of the BIOMED-2 concerted action BHM4-CT 98-3936. Leukemia. 2007. 21:201–206.
Article17. Halldórsdóttir AM, Zehnbauer BA, Burack WR. Application of BIOMED-2 clonality assays to formalin-fixed paraffin embedded follicular lymphoma specimens: superior performance of the IGK assays compared to IGH for suboptimal specimens. Leuk Lymphoma. 2007. 48:1338–1343.
Article18. Smilevska T, Tsakou E, Hadzidimitriou A, Bikos V, Stavroyianni N, Laoutaris N, et al. Immunoglobulin kappa gene repertoire and somatic hypermutation patterns in follicular lymphoma. Blood Cells Mol Dis. 2008. 41:215–218.
Article19. Liu H, Bench AJ, Bacon CM, Payne K, Huang Y, Scott MA, et al. A practical strategy for the routine use of BIOMED-2 PCR assays for detection of B- and T-cell clonality in diagnostic haematopathology. Br J Haematol. 2007. 138:31–43.
Article20. Kros JM, Bagdi EK, Zheng P, Hop WC, Driesse MJ, Krenacs L, et al. Analysis of immunoglobulin H gene rearrangement by polymerase chain reaction in primary central nervous system lymphoma. J Neurosurg. 2002. 97:1390–1396.
Article21. Essop MF, Blakolmer K, Close PM, Manuel YE, Cornelius S. Analysis of low and high grade B-cell lymphoma subtypes using semi-nested PCR and two primer sets. Eur J Haematol. 1997. 59:136–141.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Utility of a Clonality Test for Lymphoproliferative Diseases in Koreans Using the BIOMED-2 PCR Assay
- A Case of Lambda-expressing Pulmonary MALT Lymphoma with Dual Clonal Rearrangements of Kappa and Lambda Immunoglobulin Light Chain Gene
- Application of Hot Start PCR Method in PCR-based Preimplantation Genetic Diagnosis
- Comparative Assessment of Diagnostic Performance of Cytochrome Oxidase Multiplex PCR and 18S rRNA Nested PCR
- Evaluation of Multiplex PCR Assay Using Dual Priming Oligonucleotide System for Detection Mutation in the Duchenne Muscular Dystrophy Gene