Lab Med Online.
2011 Oct;1(4):184-189. 10.3343/lmo.2011.1.4.3.
Evaluation of Rapid Antigen Test for the Detection of Norovirus Infection: Comparison with ELISA and Real Time Quantitative Reverse Transcription PCR Assays
- Affiliations
-
- 1Department of Laboratory Medicine, Hallym University College of Mecidine, Seoul, Korea. hskim0901@empal.com
- 2Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 1993635
- DOI: http://doi.org/10.3343/lmo.2011.1.4.3
Abstract
- BACKGROUND
Norovirus is a leading cause of epidemic and sporadic acute gastroenteritis worldwide. Because of the rapid transmission of the virus, early detection is important to prevent outbreak of norovirus infection. To evaluate the performance of a newly introduced rapid antigen test for detecting human norovirus in stool specimens, we compared it with the established ELISA test and real time quantitative reverse transcription PCR (qRT-PCR).
METHODS
One hundred and eighty-four stool samples were analyzed by rapid antigen test (Denka-Seiken, Japan), ELISA (R-Biopharm, Germany), and qRT-PCR (R-Biopharm, Germany). Overall percent agreement, percent positive agreement (PPA), and percent negative agreement (NPA) of the rapid antigen test in comparison with ELISA and qRT-PCR were obtained.
RESULTS
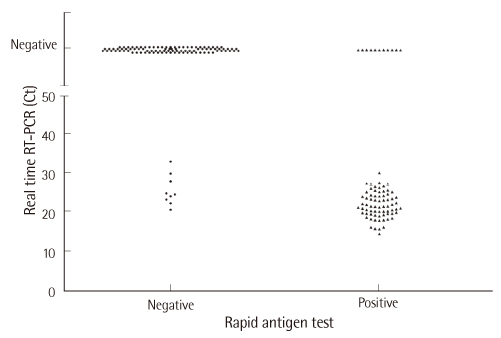
Positive rates of rapid antigen test, ELISA, and qRT-PCR were 44.0% (81/184), 51.6% (95/184), and 42.9% (79/184), respectively. Seventy samples (38.0%) showed all positive, and 86 samples (46.7%) showed all negative results by three methods. Overall percent agreement of three methods was 84.8% (156/184). Overall percent agreement, PPA, and NPA of the rapid antigen test in comparison with qRT-PCR were 89.1%, 88.6%, and 89.5%, respectively, and those of the rapid antigen test in comparison with ELISA were 90.2%, 83.2%, and 97.8%, respectively. Total procedure of the rapid antigen test was finished within 20 min.
CONCLUSIONS
Rapid antigen test was easier and quicker to perform, and showed high agreement rates with ELISA and qRT-PCR. This test may be useful for rapid screening of norovirus infection.
Keyword
MeSH Terms
Figure
Reference
-
1. Division of Epidemic Intelligence Service, Korea Centers for Disease Control and Prevention. Epidemic investigation of group diarrhea in the metropolitan area spread by a large meal service provider. Annual report on the epidemiologic investigation of infectious diseases. 2006. 153–159.2. Division of Epidemic Intelligence Service, Korea Centers for Disease Control and Prevention. Epidemic investigation on Norwalk virus that caused food poisoning in Seoul. Annual report on the epidemiologic investigation of infectious diseases. 2003. 103–113.3. Jee YM. Epidemiology of norovirus infection in Korea. Korean J Pediatr Infect Dis. 2007. 14:17–24.
Article4. Lee JI, Park SH, Kim MS, Oh YH, Yu IS, Choi BH, et al. Surveillance of acute gastroenteritis in Seoul, Korea, during May 2004 and June 2007. J Bacteriol Virol. 2009. 39:363–371.
Article5. Chung JY, Han TH, Park SH, Kim SW, Hwang ES. Detection of GII-4/2006b variant and recombinant noroviruses in children with acute gastroenteritis, South Korea. J Med Virol. 2010. 82:146–152.
Article6. Park DJ, Kim JS, Park JY, Kim HS, Song W, Kim HS, et al. Epidemiological analysis of norovirus infection between March 2007 and February 2010. Korean J Lab Med. 2010. 30:647–653.
Article7. Vinjé J, Hamidjaja RA, Sobsey MD. Development and application of a capsid VP1 (region D) based reverse transcription PCR assay for genotyping of genogroup I and II noroviruses. J Virol Methods. 2004. 116:109–117.
Article8. Atmar RL, Estes MK. Diagnosis of noncultivatable gastroenteritis viruses, the human caliciviruses. Clin Microbiol Rev. 2001. 14:15–37.
Article9. Ando T, Noel JS, Fankhauser RL. Genetic classification of "Norwalk-like viruses". J Infect Dis. 2000. 181:Suppl 2. S336–S348.
Article10. Bull RA, Tu ET, McIver CJ, Rawlinson WD, White PA. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J Clin Microbiol. 2006. 44:327–333.
Article11. Fankhauser RL, Monroe SS, Noel JS, Humphrey CD, Bresee JS, Parashar UD, et al. Epidemiologic and molecular trends of "Norwalk-like viruses" associated with outbreaks of gastroenteritis in the United States. J Infect Dis. 2002. 186:1–7.
Article12. Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006. 346:312–323.
Article13. Castriciano S, Luinstra K, Petrich A, Smieja M, Lee C, Jang D, et al. Comparison of the RIDASCREEN® norovirus enzyme immunoassay to IDEIA NLV GI/GII by testing stools also assayed by RT-PCR and electron microscopy. J Virol Methods. 2007. 141:216–219.
Article14. Bruins MJ, Wolfhagen MJ, Schirm J, Ruijs GJ. Evaluation of a rapid immunochromatographic test for the detection of norovirus in stool samples. Eur J Clin Microbiol Infect Dis. 2010. 29:741–743.
Article15. Kirby A, Gurgel RQ, Dove W, Vieira SC, Cunliffe NA, Cuevas LE. An evaluation of the RIDASCREEN and IDEIA enzyme immunoassays and the RIDAQUICK immunochromatographic test for the detection of norovirus in faecal specimens. J Clin Virol. 2010. 49:254–257.
Article16. Clinical and Laboratory Standards Institute. CLSI document EP12-A2. User protocol for evaluation of qualitative test performance. 2008. approved guideline-second edition. Wayne, PA: Clinical and Laboratory Standards Institute.17. Gray JJ, Kohli E, Ruggeri FM, Vennema H, Sánchez-Fauquier A, Schreier E, et al. European multicenter evaluation of commercial enzyme immunoassays for detecting norovirus antigen in fecal samples. Clin Vaccine Immunol. 2007. 14:1349–1355.
Article18. Okitsu-Negishi S, Okame M, Shimizu Y, Phan TG, Tomaru T, Kamijo S, et al. Detection of norovirus antigens from recombinant virus-like particles and stool samples by a commercial norovirus enzyme-linked immunosorbent assay kit. J Clin Microbiol. 2006. 44:3784–3786.
Article19. Barreira DM, Ferreira MS, Fumian TM, Checon R, de Sadovsky AD, Leite JP, et al. Viral load and genotypes of noroviruses in symptomatic and asymptomatic children in Southeastern Brazil. J Clin Virol. 2009. 47:60–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Rapid Antigen Test and Real-Time Reverse Transcription PCR for the Detection of Influenza B Virus
- Evaluation of the Efficacies of Rapid Antigen Test, Multiplex PCR, and Real-time PCR for the Detection of a Novel Influenza A (H1N1) Virus
- Accuracy and Cross-Reactivity of Rapid Diagnostic Tests for Norovirus and Rotavirus in a Real Clinical Setting
- Comparison of Rapid Antigen Test and Real-Time Reverse Transcriptase PCR for Diagnosing Novel Swine Influenza A (H1N1)
- Evaluation of two Real-Time PCR Assays for the Detection of Chlamydia trachomatis