Korean J Urol.
2014 Jan;55(1):52-56. 10.4111/kju.2014.55.1.52.
Clinical Effects of Discontinuing 5-Alpha Reductase Inhibitor in Patients With Benign Prostatic Hyperplasia
- Affiliations
-
- 1Department of Urology, Yonsei University Wonju College of Medicine, Wonju, Korea. chc7174@yonsei.ac.kr
- KMID: 1988453
- DOI: http://doi.org/10.4111/kju.2014.55.1.52
Abstract
- PURPOSE
To assess changes in lower urinary tract symptoms (LUTS), prostate volume, and serum prostate-specific antigen (PSA) after discontinuation of 5-alpha reductase inhibitor (5ARI) combination therapy in patients with benign prostatic hyperplasia (BPH).
MATERIALS AND METHODS
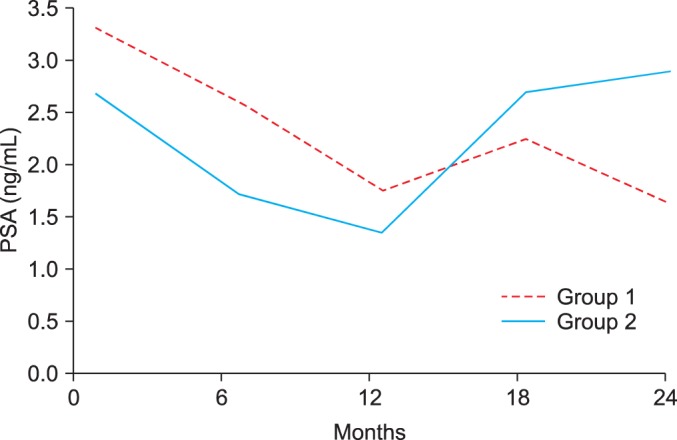
From December 2003 to December 2012, data were collected retrospectively from 81 men more than 40 years of age with moderate to severe BPH symptoms (International Prostate Symptom Score [IPSS]> or =8). The men were classified into group 1 (n=42) and group 2 (n=39) according to the use of 5ARI therapy. A combination of dutasteride 0.5 mg with tamsulosin 0.2 mg was given daily to all patients for 1 year. For the next 1 year, group 1 (n=42) received the combination therapy and group 2 (n=39) received tamsulosin 0.2 mg monotherapy only. The IPSS, prostate volume, and PSA level were measured at baseline and at 12 and 24 months according to the use of dutasteride.
RESULTS
Discontinuation of dutasteride led to significant deterioration of LUTS, increased prostate volume, and increased PSA level. The repeated-measures analysis of variance showed that the changes in IPSS, prostate volume, and PSA level over time also differed significantly between groups 1 and 2 (p<0.001).
CONCLUSIONS
Withdrawal of 5ARI during combination therapy resulted in prostate regrowth and deterioration of LUTS. The PSA level is also affected by the use of 5ARI. Therefore, regular check-up of the IPSS and PSA level may be helpful for all patients who either continue or discontinue the use of 5ARI.
MeSH Terms
Figure
Cited by 2 articles
-
Patient's Factors Correlated with Prostate Volume Recovery after 5 Alpha Reductase Inhibitor Discontinuation
Kwibok Choi, Byounghoon Kim, In-Chang Cho, Seung Ki Min
Urogenit Tract Infect. 2018;13(3):79-83. doi: 10.14777/uti.2018.13.3.79.Clinical Course of the Benign Prostate Hyplasia Patients during the Intermittent Use of 5-Alpha Reductase Inhibitors
Kwibok Choi, Byounghoon Kim, In-Chang Cho, Seung Ki Min
Urogenit Tract Infect. 2019;14(3):93-96. doi: 10.14777/uti.2019.14.3.93.
Reference
-
1. Emberton M, Andriole GL, de la Rosette J, Djavan B, Hoefner K, Vela Navarrete R, et al. Benign prostatic hyperplasia: a progressive disease of aging men. Urology. 2003; 61:267–273. PMID: 12597928.
Article2. Jacobsen SJ, Jacobson DJ, Girman CJ, Roberts RO, Rhodes T, Guess HA, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997; 158:481–487. PMID: 9224329.
Article3. Boyle P, Roehrborn C, Harkaway R, Logie J, de la Rosette J, Emberton M. 5-Alpha reductase inhibition provides superior benefits to alpha blockade by preventing AUR and BPH-related surgery. Eur Urol. 2004; 45:620–626. PMID: 15082205.
Article4. Debruyne F, Barkin J, van Erps P, Reis M, Tammela TL, Roehrborn C, et al. Efficacy and safety of long-term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004; 46:488–494. PMID: 15363566.5. Marks LS, Andriole GL, Fitzpatrick JM, Schulman CC, Roehrborn CG. The interpretation of serum prostate specific antigen in men receiving 5alpha-reductase inhibitors: a review and clinical recommendations. J Urol. 2006; 176:868–874. PMID: 16890642.6. Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G. The long-term outcome of medical therapy for BPH. Eur Urol. 2007; 51:1522–1533. PMID: 17416456.
Article7. Roehrborn CG. 5-Alpha-reductase inhibitors prevent the progression of benign prostatic hyperplasia. Rev Urol. 2003; 5(Suppl 4):S18–S27. PMID: 16985959.8. Tanguay S, Awde M, Brock G, Casey R, Kozak J, Lee J, et al. Diagnosis and management of benign prostatic hyperplasia in primary care. Can Urol Assoc J. 2009; 3(3 Suppl 2):S92–S100. PMID: 19543429.
Article9. Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G. ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002; 60:434–441. PMID: 12350480.
Article10. Marberger M, Harkaway R, de la Rosette J. Optimising the medical management of benign prostatic hyperplasia. Eur Urol. 2004; 45:411–419. PMID: 15041103.
Article11. Nickel JC, Barkin J, Koch C, Dupont C, Elhilali M. Finasteride monotherapy maintains stable lower urinary tract symptoms in men with benign prostatic hyperplasia following cessation of alpha blockers. Can Urol Assoc J. 2008; 2:16–21. PMID: 18542722.
Article12. Roehrborn CG, Lukkarinen O, Mark S, Siami P, Ramsdell J, Zinner N. Long-term sustained improvement in symptoms of benign prostatic hyperplasia with the dual 5alpha-reductase inhibitor dutasteride: results of 4-year studies. BJU Int. 2005; 96:572–577. PMID: 16104912.
Article13. Kirby RS, Roehrborn C, Boyle P, Bartsch G, Jardin A, Cary MM, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003; 61:119–126. PMID: 12559281.
Article14. McConnell JD, Roehrborn CG, Bautista OM, Andriole GL Jr, Dixon CM, Kusek JW, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003; 349:2387–2398. PMID: 14681504.
Article15. Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab. 2004; 89:2179–2184. PMID: 15126539.16. Barkin J, Guimaraes M, Jacobi G, Pushkar D, Taylor S, van Vierssen Trip OB. Alpha-blocker therapy can be withdrawn in the majority of men following initial combination therapy with the dual 5alpha-reductase inhibitor dutasteride. Eur Urol. 2003; 44:461–466. PMID: 14499682.17. Andersen JT, Nickel JC, Marshall VR, Schulman CC, Boyle P. Finasteride significantly reduces acute urinary retention and need for surgery in patients with symptomatic benign prostatic hyperplasia. Urology. 1997; 49:839–845. PMID: 9187688.
Article18. Kaplan SA, Ghafar MA, Volpe MA, Lam JS, Fromer D, Te AE. PSA response to finasteride challenge in men with a serum PSA greater than 4 ng/ml and previous negative prostate biopsy: preliminary study. Urology. 2002; 60:464–468. PMID: 12350485.
Article19. Stoner E. The clinical effects of a 5 alpha-reductase inhibitor, finasteride, on benign prostatic hyperplasia. The Finasteride Study Group. J Urol. 1992; 147:1298–1302. PMID: 1373779.20. Jeong YB, Kwon KS, Kim SD, Kim HJ. Effect of discontinuation of 5alpha-reductase inhibitors on prostate volume and symptoms in men with BPH: a prospective study. Urology. 2009; 73:802–806. PMID: 19193422.21. Andriole GL, Marberger M, Roehrborn CG. Clinical usefulness of serum prostate specific antigen for the detection of prostate cancer is preserved in men receiving the dual 5alpha-reductase inhibitor dutasteride. J Urol. 2006; 175:1657–1662. PMID: 16600723.22. Bartsch G, Fitzpatrick JM, Schalken JA, Isaacs J, Nordling J, Roehrborn CG. Consensus statement: the role of prostate-specific antigen in managing the patient with benign prostatic hyperplasia. BJU Int. 2004; 93(Suppl 1):27–29. PMID: 15009083.
Article23. Guess HA, Gormley GJ, Stoner E, Oesterling JE. The effect of finasteride on prostate specific antigen: review of available data. J Urol. 1996; 155:3–9. PMID: 7490873.
Article24. Andriole GL, Bostwick D, Brawley OW, Gomella L, Marberger M, Montorsi F, et al. The effect of dutasteride on the usefulness of prostate specific antigen for the diagnosis of high grade and clinically relevant prostate cancer in men with a previous negative biopsy: results from the REDUCE study. J Urol. 2011; 185:126–131. PMID: 21074214.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficacy of Combination Therapy of 5 alpha -Reductase Inhibitor and of-Adrenergic Blocker in Benign Prostate Hyperplasia
- Effect of 5-alpha Reductase Inhibitor on Storage Symptoms in Patients with Benign Prostatic Hyperplasia
- The Antihyperplastic Effect of Oral Catechin Ingestion in a Rat Model of Benign Prostatic Hyperplasia
- Comparison of Clinical Efficacy of Finasteride and Dutasteride as 5-alpha Reductase Inhibitor
- alpha-Blocker Monotherapy and alpha-Blocker Plus 5-Alpha-Reductase Inhibitor Combination Treatment in Benign Prostatic Hyperplasia; 10 Years' Long-Term Results