Korean J Urol.
2014 May;55(5):349-353. 10.4111/kju.2014.55.5.349.
Unexpected Multidrug Resistance of Methicillin-Resistant Staphylococcus aureus in Urine Samples: A Single-Center Study
- Affiliations
-
- 1Department of Urology, Hanusch Krankenhaus, Vienna, Austria. andreas.lunacek@acmp.at
- 2Department of Pathology, Hanusch Krankenhaus, Vienna, Austria.
- 3Department of Urology, Medical University Innsbruck, Innsbruck, Austria.
- KMID: 1988413
- DOI: http://doi.org/10.4111/kju.2014.55.5.349
Abstract
- PURPOSE
Infections of methicillin-resistant Staphylococcus aureus (MRSA) are becoming an increasingly concerning clinical problem. The aim of this study was to assess the development of MRSA in urine cultures in a major public university-affiliated hospital and the therapeutical and hygiene-related possibilities for reducing resistance.
MATERIALS AND METHODS
This study included 243 samples from patients diagnosed with MRSA infection over a period of 6 years. An agar diffusion test measured the effects of antimicrobial agents against bacteria grown in culture. The analyses were based on the guidelines of the Clinical and Laboratory Standards Institute.
RESULTS
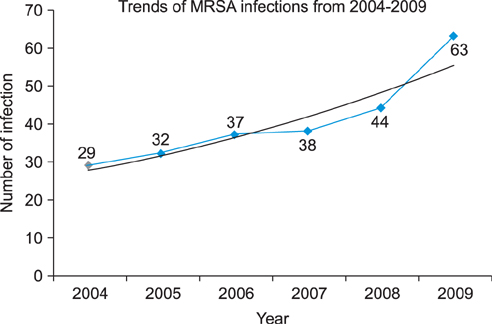
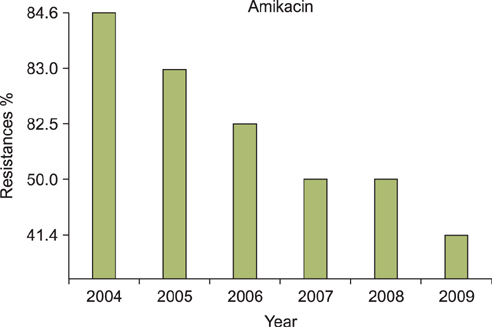
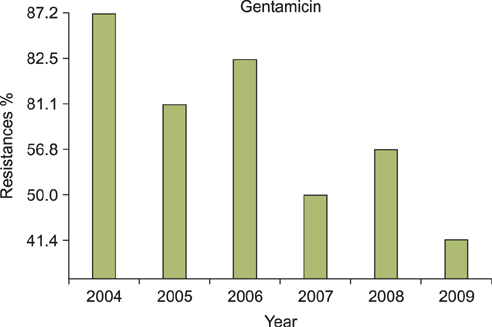
A regression analysis was performed, which showed 100% resistance to the following antibiotics throughout the entire testing period: carbapenem, cephalosporin (1st-4th generation), penicillin G, aminopenicillin, beta-lactamase, and isoxazolyl penicillin. However, a significant decrease in resistance was found for amikacin, gentamicin, clindamycin, levofloxacin, erythromycin, and mupirocin.
CONCLUSIONS
MRSA showed a decreasing trend of antimicrobial resistance, except against carbapenem, cephalosporin (1st-4th generation), penicillin G, aminopenicillin, beta-lactamase, and isoxazolyl penicillin, for which complete resistance was observed.
MeSH Terms
-
Agar
Amikacin
Anti-Bacterial Agents
Anti-Infective Agents
Bacteria
beta-Lactamases
Clindamycin
Diffusion
Drug Resistance, Multiple*
Erythromycin
Gentamicins
Humans
Levofloxacin
Methicillin-Resistant Staphylococcus aureus*
Mupirocin
Penicillin G
Penicillins
Agar
Amikacin
Anti-Bacterial Agents
Anti-Infective Agents
Clindamycin
Erythromycin
Gentamicins
Mupirocin
Penicillin G
Penicillins
beta-Lactamases
Figure
Reference
-
1. Mehndiratta PL, Gur R, Saini S, Bhalla P. Staphylococcus aureus phage types and their correlation to antibiotic resistance. Indian J Pathol Microbiol. 2010; 53:738–741.2. Loomba PS, Taneja J, Mishra B. Methicillin and vancomycin resistant S. aureus in hospitalized patients. J Glob Infect Dis. 2010; 2:275–283.3. Chihara S, Popovich KJ, Weinstein RA, Hota B. Staphylococcus aureus bacteriuria as a prognosticator for outcome of Staphylococcus aureus bacteremia: a case-control study. BMC Infect Dis. 2010; 10:225.4. Lee BK, Crossley K, Gerding DN. The association between Staphylococcus aureus bacteremia and bacteriuria. Am J Med. 1978; 65:303–306.5. Muder RR, Brennen C, Rihs JD, Wagener MM, Obman A, Stout JE, et al. Isolation of Staphylococcus aureus from the urinary tract: association of isolation with symptomatic urinary tract infection and subsequent staphylococcal bacteremia. Clin Infect Dis. 2006; 42:46–50.6. Pfaller MA, Acar J, Jones RN, Verhoef J, Turnidge J, Sader HS. Integration of molecular characterization of microorganisms in a global antimicrobial resistance surveillance program. Clin Infect Dis. 2001; 32:Suppl 2. S156–S167.7. Stevens DL, Herr D, Lampiris H, Hunt JL, Batts DH, Hafkin B. Linezolid versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus infections. Clin Infect Dis. 2002; 34:1481–1490.8. Diller R, Sonntag AK, Mellmann A, Grevener K, Senninger N, Kipp F, et al. Evidence for cost reduction based on pre-admission MRSA screening in general surgery. Int J Hyg Environ Health. 2008; 211:205–212.9. Ando E, Monden K, Mitsuhata R, Kariyama R, Kumon H. Biofilm formation among methicillin-resistant Staphylococcus aureus isolates from patients with urinary tract infection. Acta Med Okayama. 2004; 58:207–214.10. Abe Y, Shigemura K, Yoshida H, Fujisawa M, Arakawa S. Risk factors for anti-MRSA drug resistance. Int J Antimicrob Agents. 2012; 40:423–426.11. Orrett FA, Land M. Methicillin-resistant Staphylococcus aureus prevalence: current susceptibility patterns in Trinidad. BMC Infect Dis. 2006; 6:83.12. Riedl CR, Plas E, Hubner WA, Zimmerl H, Ulrich W, Pfluger H. Bacterial colonization of ureteral stents. Eur Urol. 1999; 36:53–59.13. Gotz F. Staphylococcus and biofilms. Mol Microbiol. 2002; 43:1367–1378.14. Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002; 15:167–193.15. Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004; 2:95–108.16. Currie K, Knussen C, Price L, Reilly J. Methicillin-resistant Staphylococcus aureus screening as a patient safety initiative: using patients' experiences to improve the quality of screening practices. J Clin Nurs. 2014; 23:221–231.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Multidrug Resistant Patterns and Associated - genes of Methicillin Resistant Staphylococcus aureus ( MRSA ) Isolated from Clinical Specimens
- A statistical analysis of methicillin-resistant staphylococcus aureus
- A case of multiple furunculosis caused by methicillin-resistant staphylococcs aureus
- A Survey for Methicillin - Resistant Staphylococcus Aureus
- Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)