J Adv Prosthodont.
2015 Feb;7(1):56-61. 10.4047/jap.2015.7.1.56.
Influence of shape and finishing on the corrosion of palladium-based dental alloys
- Affiliations
-
- 1Department of Dental Materials Science, ACTA, University of Amsterdam and VU University Amsterdam, Nederland. A.Milheiro@acta.nl
- KMID: 1974892
- DOI: http://doi.org/10.4047/jap.2015.7.1.56
Abstract
- PURPOSE
The purpose of this study was to evaluate the effects of the surface treatment and shape of the dental alloy on the composition of the prosthetic work and its metallic ion release in a corrosive medium after casting.
MATERIALS AND METHODS
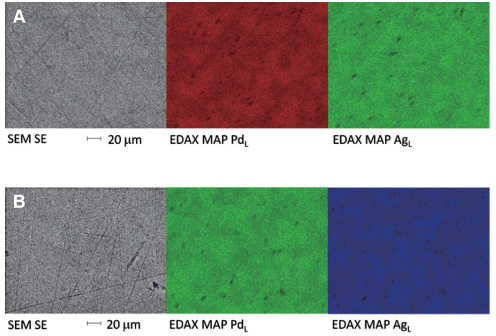
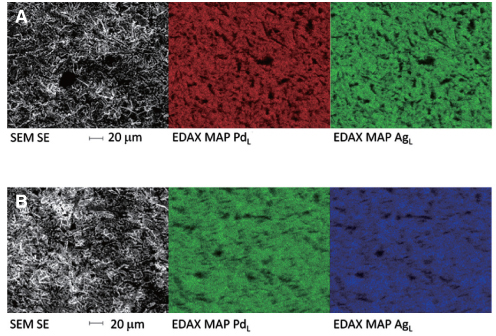
Orion Argos (Pd-Ag) and Orion Vesta (Pd-Cu) were used to cast two crowns and two disks. One of each was polished while the other was not. Two as-received alloys were also studied making a total of 5 specimens per alloy type. The specimens were submersed for 7 days in a lactic acid/sodium chloride solution (ISO standard 10271) and evaluated for surface structure characterization using SEM/EDAX. The solutions were quantitatively analysed for the presence of metal ions using ICP-MS and the results were statistically analysed with one-way ANOVA and a Tukey post-hoc test.
RESULTS
Palladium is released from all specimens studied (range 0.06-7.08 microg.cm(-2).week(-1)), with the Pd-Cu alloy releasing the highest amounts. For both types of alloys, ion release of both disk and crown pairs were statistically different from the as-received alloy except for the Pd-Ag polished crown (P>.05). For both alloy type, disk-shaped pairs and unpolished specimens released the highest amounts of Pd ions (range 0.34-7.08 microg.cm(-2).week(-1)). Interestingly, in solutions submerged with cast alloys trace amounts of unexpected elements were measured.
CONCLUSION
Shape and surface treatment influence ion release from dental alloys; polishing is a determinant factor. The release rate of cast and polished Pd alloys is between 0.06-0.69 microg.cm(-2).week(-1), which is close to or exceeding the EU Nickel Directive 94/27/EC compensated for the molecular mass of Pd (0.4 microg.cm(-2).week(-1)). The composition of the alloy does not represent the element release, therefore we recommend manufacturers to report element release after ISO standard corrosion tests beside the original composition.
Keyword
MeSH Terms
Figure
Reference
-
1. Faurschou A, Menné T, Johansen JD, Thyssen JP. Metal allergen of the 21st century-a review on exposure, epidemiology and clinical manifestations of palladium allergy. Contact Dermatitis. 2011; 64:185–195.2. Kielhorn J, Melber C, Keller D, Mangelsdorf I. Palladium-a review of exposure and effects to human health. Int J Hyg Environ Health. 2002; 205:417–432.3. Wise EM, Eash JT. The Role of the Platinum Metals in Dental Alloys. III. The Influence of Platinum and Palladium and Heat Treatment upon the Microstructure and Constitution of Basic Alloys. Trans AIME Inst Metals Div. 1933; 102:270–307.4. Goodacre CJ. Palladium-silver alloys: a review of the literature. J Prosthet Dent. 1989; 62:34–37.5. Wataha JC. Biocompatibility of dental casting alloys: a review. J Prosthet Dent. 2000; 83:223–234.6. Schmalz G, Garhammer P. Biological interactions of dental cast alloys with oral tissues. Dent Mater. 2002; 18:396–406.7. WHO. International Programme on Chemical Safety (IPCS) Environmental Health Criteria (EHC) 226. Organization WH. Palladium. Geneva: 2002. p. 202.8. Manaranche C, Hornberger H. A proposal for the classification of dental alloys according to their resistance to corrosion. Dent Mater. 2007; 23:1428–1437.9. Endo K, Ohno H, Matsuda K, Asakura S. Electrochemical and surface studies on the passivity of a dental Pd-based casting alloy in alkaline sulphide solution. Corrosion Science. 2003; 45:1491–1504.10. Wataha JC, Lockwood PE. Release of elements from dental casting alloys into cell-culture medium over 10 months. Dent Mater. 1998; 14:158–163.11. Mülders C, Darwish M, Holze R. The influence of alloy composition and casting procedure upon the corrosion behaviour of dental alloys: an in vitro study. J Oral Rehabil. 1996; 23:825–831.12. Wataha JC, Craig RG, Hanks CT. The release of elements of dental casting alloys into cell-culture medium. J Dent Res. 1991; 70:1014–1018.13. Wataha JC, Lockwood PE, Khajotia SS, Turner R. Effect of pH on element release from dental casting alloys. J Prosthet Dent. 1998; 80:691–698.14. Wataha JC, Nelson SK, Lockwood PE. Elemental release from dental casting alloys into biological media with and without protein. Dent Mater. 2001; 17:409–414.15. Viennot S, Lissac M, Malquarti G, Dalard F, Grosgogeat B. Influence of casting procedures on the corrosion resistance of clinical dental alloys containing palladium. Acta Biomater. 2006; 2:321–330.16. Cai Z, Vermilyea SG, Brantley WA. In vitro corrosion resistance of high-palladium dental casting alloys. Dent Mater. 1999; 15:202–210.17. Horasawa N, Marek M. The effect of recasting on corrosion of a silver-palladium alloy. Dent Mater. 2004; 20:352–357.18. ISO 10271:2011. Dentistry-Corrosion test methods for metallic materials. Geneva; Switzerland: International Standards Organization (ISO);2011.19. Nitta SV, Clark WA, Brantley WA, Grylls RJ, Cai Z. TEM analysis of tweed structure in high-palladium dental alloys. J Mater Sci Mater Med. 1999; 10:513–517.20. Wataha JC, Malcolm CT. Effect of alloy surface composition on release of elements from dental casting alloys. J Oral Rehabil. 1996; 23:583–589.21. Sarkar NK, Berzins DW, Prasad A. Dealloying and electroformation in high-Pd dental alloys. Dent Mater. 2000; 16:374–379.22. Kaneko T, Hattori M, Hasegawa K, Yoshinari M, Kawada E, Oda Y. Influence of finishing on the electrochemical properties of dental alloys. Bull Tokyo Dent Coll. 2000; 41:49–57.23. Berzins DW, Kawashima I, Graves R, Sarkar NK. Heat treatment effects on electrochemical corrosion parameters of high-Pd alloys. J Mater Sci Mater Med. 2008; 19:335–341.24. Carr AB, Brantley WA. New high-palladium casting alloys: 1. Overview and initial studies. Int J Prosthodont. 1991; 4:265–275.25. Carr AB, Cai Z, Brantley WA, Mitchell JC. New high-palladium casting alloys: Part 2. Effects of heat treatment and burnout temperature. Int J Prosthodont. 1993; 6:233–241.26. Brantley WA, Cai Z, Carr AB, Mitchell JC. Metallurgical Structures of as-Cast and Heat-Treated High-Palladium Dental Alloys. Cells Mater. 1993; 3:103–114.27. Tuna SH, Pekmez NO, Keyf F, Canli F. The influence of the pure metal components of four different casting alloys on the electrochemical properties of the alloys. Dent Mater. 2009; 25:1096–1103.28. Pistoor FH, Kapsenberg ML, Bos JD, Meinardi MM, von Blomberg ME, Scheper RJ. Cross-reactivity of human nickelreactive T-lymphocyte clones with copper and palladium. J Invest Dermatol. 1995; 105:92–95.29. Moulon C, Weltzien HU. Human Nickel-Specific T-Cell Clones Cross-React with Other Metal Sensitizers - Implications for Associated Contact Sensitivities. J Invest Dermatol. 1995; 105:451.30. Hindsén M, Spirén A, Bruze M. Cross-reactivity between nickel and palladium demonstrated by systemic administration of nickel. Contact Dermatitis. 2005; 53:2–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of repeated porcelain firings on corrosion resistance of different dental alloys
- A STUDY OF INTERFACE AND CORROSION BEHAVIOR BETWEEN IMPLANT ABUTMENT AND CASTING GOLD ALLOY
- A STUDY OF ION BEAM ASSISTED DEPOSITION(IBAD) OF TiN ON Ni-Cr Be ALLOY FOR SURFACE CHARACTERISTIC
- The effect of solder and laser weld on corrosion of dental alloys
- Surface roughness changes caused by the galvanic corrosion between a titanium abutment and base metal alloy