Cancer Res Treat.
2008 Dec;40(4):197-201.
Altered Expression of Smad Proteins in T or NK-cell Lymphomas
- Affiliations
-
- 1Department of Pathology, Dankook University College of Medicine, Cheonan, Korea. jaihyang@yahoo.co.kr
Abstract
-
PURPOSE: Smad proteins mediate cellular signaling through the transforming growth factor-beta family (TGF-beta s). Smads 2 and 3 transmit signals from TGF-beta, and Smad4 is a common mediator, as well. However, little is known concerning the expression patterns of Smads in lymphoid tissue.
MATERIALS AND METHODS
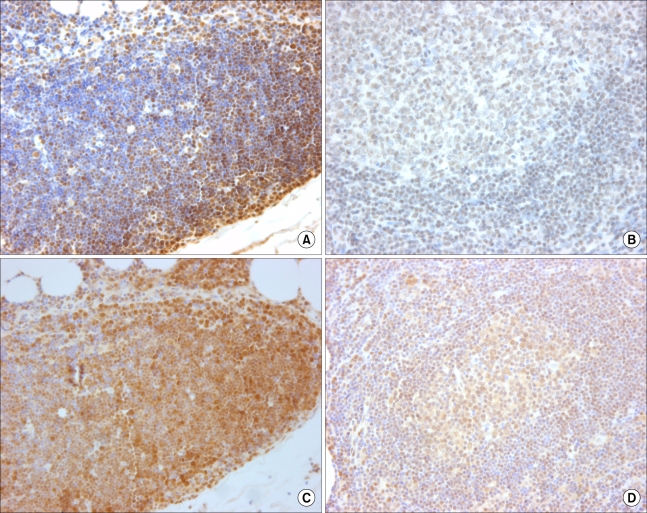
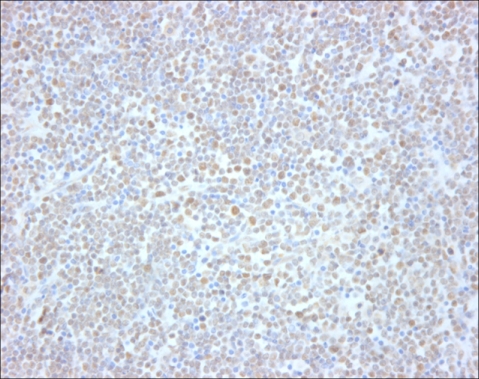
Immunohistochemistry for Smad3 and Smad4 was performed on paraffin-embedded tissue sections collected from 26 T- or NK-cell lymphomas.
RESULTS
Nearly all cells in germinal centers were positive for Smad3, and more than 50% of paracortical cells were positive for Smad3 in reactive lymphoid tissue. When Smad4 immunostaining was conducted, nearly all the cells in the germinal centers showed diffuse cytoplasmic staining, and most of them exhibited nuclear positivity, as well. In addition, more than 50% of the cells in the paracortex were positive for Smad4. Furthermore, the Smad3 staining pattern was preserved in all malignant lymphomas, but four of these cases (15%) exhibited decreased expression of Smad4. All lymphoblastic lymphomas showed strong positivity in most of tumor cells, but one unspecified peripheral lymphoma, two nasal NK/T cell lymphomas, and one anaplastic large cell lymphoma were negative for Smad4.
CONCLUSIONS
These results suggest that TGF-beta-specific Smads may be actively involved in signal transduction in lymphoid organs and that Smad-mediated TGF-beta signaling pathways are operative in malignant lymphoma. In addition, loss of Smad4 expression might be associated with development of some T-cell lymphomas.
Keyword
MeSH Terms
Figure
Reference
-
1. de Caestecker MP, Yahata T, Wang D, Parks WT, Huang S, Hill CS, et al. The Smad4 activation domain (SAD) is a proline-rich, p300-dependent transcriptional activation domain. J Biol Chem. 2000; 275:2115–2122. PMID: 10636916.
Article2. Flanders KC, Kim ES, Roberts AB. Immunohistochemical expression of Smads 1-6 in the 15-day gestation mouse embryo: signaling by BMPs and TGF-betas. Dev Dyn. 2001; 220:141–154. PMID: 11169847.3. Larisch-Bloch S, Danielpour D, Roche NS, Lotan R, Hsing AY, Kerner H, et al. Selective loss of the transforming growth factor-beta apoptotic signaling pathway in mutant NRP-154 rat prostatic epithelial cells. Cell Growth Differ. 2000; 11:1–10. PMID: 10672898.4. Patil S, Wildey GM, Brown TL, Choy L, Derynck R, Howe PH. Smad7 is induced by CD40 and protects WEHI 231 B-lymphocytes from transforming growth factor-beta-induced growth inhibition and apoptosis. J Biol Chem. 2000; 275:38363–38370. PMID: 10995749.5. Miyazono K. TGF-beta receptors and signal transduction. Int J Hematol. 1997; 65:97–104. PMID: 9071813.6. Schiemann WP, Pfeifer WM, Levi E, Kadin ME, Lodish HF. A deletion in the gene for transforming growth factor beta type I receptor abolishes growth regulation by transforming growth factor beta in a cutaneous T-cell lymphoma. Blood. 1999; 94:2854–2861. PMID: 10515889.7. Kadin ME, Cavaille-Coll MW, Gertz R, Massague J, Cheifetz S, George D. Loss of receptors for transforming growth factor beta in human T-cell malignancies. Proc Natl Acad Sci USA. 1994; 91:6002–6006. PMID: 8016105.
Article8. Knaus PI, Lindemann D, DeCoteau JF, Perlman R, Yankelev H, Hille M, et al. A dominant inhibitory mutant of the type II transforming growth factor beta receptor in the malignant progression of a cutaneous T-cell lymphoma. Mol Cell Biol. 1996; 16:3480–3489. PMID: 8668164.
Article9. Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998; 92:645–656. PMID: 9506519.10. Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998; 94:703–714. PMID: 9753318.
Article11. Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002; 2:46–53. PMID: 11905837.12. Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007; 7:443–453. PMID: 17525753.13. Rosendahl A, Speletas M, Leandersson K, Ivars F, Sideras P. Transforming growth factor-beta- and Activin-Smad signaling pathways are activated at distinct maturation stages of the thymopoiesis. Int Immunol. 2003; 15:1401–1414. PMID: 14645149.14. Djaborkhel R, Tvrdik D, Eckschlager T, Raska I, Muller J. Cyclin A down-regulation in TGFbeta1-arrested follicular lymphoma cells. Exp Cell Res. 2000; 261:250–259. PMID: 11082295.15. Hsu SM, Lin J, Xie SS, Hsu PL, Rich S. Abundant expression of transforming growth factor-beta 1 and -beta 2 by Hodgkin's Reed-Sternberg cells and by reactive T lymphocytes in Hodgkin's disease. Hum Pathol. 1993; 24:249–255. PMID: 7681031.16. Go JH. Decreased expression of TGF-β type 2 receptor in primary B-cell lymphomas of the stomach. Pathol Res Pract. 2002; 198:333–337. PMID: 12092769.17. Kadin ME, Agnarsson BA, Ellingsworth LR, Newcom SR. Immunohistochemical evidence of a role for transforming growth factor beta in the pathogenesis of nodular sclerosing Hodgkin's disease. Am J Pathol. 1990; 136:1209–1214. PMID: 2356855.18. Kim SJ, Letterio J. Transforming growth factor-beta signaling in normal and malignant hematopoiesis. Leukemia. 2003; 17:1731–1737. PMID: 12970772.19. Hu X, Zuckerman KS. Transforming growth factor: signal transduction pathways, cell cycle mediation, and effects on hematopoiesis. J Hematother Stem Cell Res. 2001; 10:67–74. PMID: 11276360.
Article20. Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, et al. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness. EMBO J. 1999; 18:1280–1291. PMID: 10064594.21. Go JH. Expression Pattern of smad proteins in diffuse large b-cell lymphomas. Korean J Pathol. 2004; 38:301–305.22. Wolfraim LA, Fernandez TM, Mamura M, Fuller WL, Kumar R, Cole DE, et al. Loss of Smad3 in acute T-cell lymphoblastic leukemia. N Engl J Med. 2004; 351:552–559. PMID: 15295048.
Article23. Chi SG. Transcriptional Regulation of Oncogenes by TGF-β1. Cancer Res Treat. 2003; 35(Suppl 1):12.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression Pattern of Smad Proteins in Diffuse Large B-cell Lymphomas
- A Case of Nasal CD56+ NK/T Cell Lymphoma Mimicking Cellulitis which Developed after Persistent Orbital Swelling
- Upregulated Neuro-oncological Ventral Antigen 1 (NOVA1) Expression Is Specific to Mature and Immature T- and NK-Cell Lymphomas
- Expression of Minichromosome Maintenance Protein 7 and Smad 4 in Squamous Cell Carcinoma of the Esophagus
- Granzyme B immunoreactivity in T/natural killer cell lymphomas