Cancer Res Treat.
2008 Dec;40(4):172-177.
Clinical Characteristics and Treatment Results of Pediatric Osteosarcoma: The Role of High Dose Chemotherapy with Autologous Stem Cell Transplantation
- Affiliations
-
- 1Division of Hematology/Oncology, Department of Pediatrics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. hyshin@snu.ac.kr
- 2Department of Orthopedics, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pathology, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Radiology, Seoul National University College of Medicine, Seoul, Korea.
Abstract
-
PURPOSE: In this study, we investigated the clinical characteristics and treatment results of osteosarcoma during the past 7 years, and evaluated the role of high dose chemotherapy (HDCT) with autologous stem cell transplantation (ASCT).
MATERIALS AND METHODS
We retrospectively analyzed the clinical data of patients who were diagnosed as osteosarcoma at our center from January, 2000 to December, 2007.
RESULTS
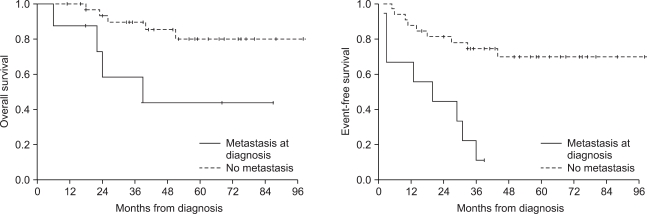
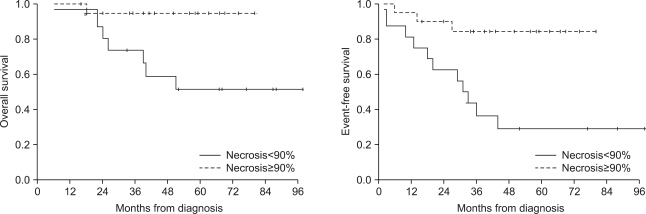
The 5-year overall survival and event-free survival of the patients were 72.6% and 55.9%, respectively. Seventeen (41.5%) patients showed disease progression during treatment or relapse after the end of treatment. The patients who had metastasis at diagnosis or who had a lower grade of necrosis after neoadjuvant chemotherapy showed decreased overall and event-free survival. Four patients received ASCT after HDCT, and 3 of them are alive without disease.
CONCLUSIONS
The patients who relapsed or had refractory osteosarcoma or who had metastasis at diagnosis or a lower grade of necrosis after neoadjuvant chemotherapy showed poor prognosis. HDCT with ASCT could be an alternative treatment option for these patients.
MeSH Terms
Figure
Reference
-
1. Marcove RC, Mike V, Hajek JV, Levin AG, Hutter RV. Osteogenic sarcoma under the age of twenty-one. A review of one hundred and forty-five operative cases. J Bone Joint Surg Am. 1970; 52:411–423. PMID: 5269156.2. Bacci G, Picci P, Ferrari S, Ruggieri P, Casadei R, Tienghi A, et al. Primary chemotherapy and delayed surgery for nonmetastatic osteosarcoma of the extremities. Results in 164 patients preoperatively treated with high doses of methotrexate followed by cisplatin and doxorubicin. Cancer. 1993; 72:3227–3238. PMID: 8242546.
Article3. Bacci G, Picci P, Ruggieri P, Mercuri M, Avella M, Capanna R, et al. Primary chemotherapy and delayed surgery (neoadjuvant chemotherapy) for osteosarcoma of the extremities. The Istituto Rizzoli Experience in 127 patients treated preoperatively with intravenous methotrexate (high versus moderate doses) and intraarterial cisplatin. Cancer. 1990; 65:2539–2553. PMID: 2337871.
Article4. Meyers PA, Gorlick R, Heller G, Casper E, Lane J, Huvos AG, et al. Intensification of preoperative chemotherapy for osteogenic sarcoma: results of the Memorial Sloan-Kettering (T12) protocol. J Clin Oncol. 1998; 16:2452–2458. PMID: 9667263.
Article5. Saeter G, Alvegard TA, Elomaa I, Stenwig AE, Holmstrom T, Solheim OP. Treatment of osteosarcoma of the extremities with the T-10 protocol, with emphasis on the effects of preoperative chemotherapy with single-agent high-dose methotrexate: a Scandinavian Sarcoma Group study. J Clin Oncol. 1991; 9:1766–1775. PMID: 1717666.
Article6. Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002; 20:776–790. PMID: 11821461.
Article7. Glasser DB, Lane JM, Huvos AG, Marcove RC, Rosen G. Survival, prognosis, and therapeutic response in osteogenic sarcoma. The Memorial Hospital experience. Cancer. 1992; 69:698–708. PMID: 1730120.
Article8. Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol. 2007; 19:341–346. PMID: 17545797.
Article9. Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006; 32:423–436. PMID: 16860938.
Article10. Fagioli F, Aglietta M, Tienghi A, Ferrari S, Brach del Prever A, Vassallo E, et al. High-dose chemotherapy in the treatment of relapsed osteosarcoma: an Italian sarcoma group study. J Clin Oncol. 2002; 20:2150–2156. PMID: 11956277.
Article11. Kasper B, Lehnert T, Bernd L, Mechtersheimer G, Goldschmidt H, Ho AD, et al. High-dose chemotherapy with autologous peripheral blood stem cell transplantation for bone and soft-tissue sarcomas. Bone Marrow Transplant. 2004; 34:37–41. PMID: 15170176.
Article12. Bielack SS, Bieling P, Erttmann R, Winkler K. Intraarterial chemotherapy for osteosarcoma: does the result really justify the effort? Cancer Treat Res. 1993; 62:85–92. PMID: 8096763.
Article13. Jaffe N, Prudich J, Knapp J, Wang YM, Bowman R, Cangir A, et al. Treatment of primary osteosarcoma with intra-arterial and intravenous high-dose methotrexate. J Clin Oncol. 1983; 1:428–431. PMID: 6607977.
Article14. Choi HS, Kang HJ, Lee JA, Han HJ, Park HJ, Seong KW, et al. Treatment Result of Pediatric Osteosarcoma with Intraarterial Cisplatin. J Korean Cancer Assoc. 1998; 30:169–177.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Evolving Role of Myeloablative Chemotherapy with Stem Cell Transplantation for the Treatment of Autoimmune Disease
- A Case of Autologous Cord Blood Stem Cell Transplantationin Stage IV Neuroblastoma
- Triple High Dose Chemotherapy Followed by Autologous Stem Cell Transplantation for Pediatric Neuroblastoma
- Autoimmune Diseases after Autologous Hematopoietic Stem Cell Transplantation in Patients with Non-Hodgkin's Lymphoma
- Effect of Fixed Dose of G-CSF on Mobilization and Engraftment of Peripheral Blood Stem Cells in High Dose Chemotherapy