J Clin Neurol.
2015 Jul;11(3):227-233. 10.3988/jcn.2015.11.3.227.
The Etiological Spectrum of Acute Sensory Myelitis
- Affiliations
-
- 1Department of Neurology, Ewha Womans University Graduate School of Medicine, Seoul, Korea. pkd1165@ewha.ac.kr
- 2Department of Neurology, Myongji Hospital, Goyang, Korea.
- 3Department of Neurology, National Cancer Center, Goyang, Korea.
- KMID: 1894554
- DOI: http://doi.org/10.3988/jcn.2015.11.3.227
Abstract
- BACKGROUND AND PURPOSE
Acute myelitis patients exhibiting only sensory deficits upon initial presentation are not commonly encountered in clinical practice, but they definitely exist. Since acute sensory myelitis has not been investigated previously, this study evaluated the etiological spectrum of the condition with the aim of describing the clinical characteristics thereof.
METHODS
Patients with acute myelitis who presented at the Ewha Womans University Medical Center (during 1999-2012) and the National Cancer Center (during 2005-2014) with only sensory symptoms as first clinical features were enrolled in this study. Their medical records, electrophysiological and laboratory data, and MRI findings were analyzed retrospectively.
RESULTS
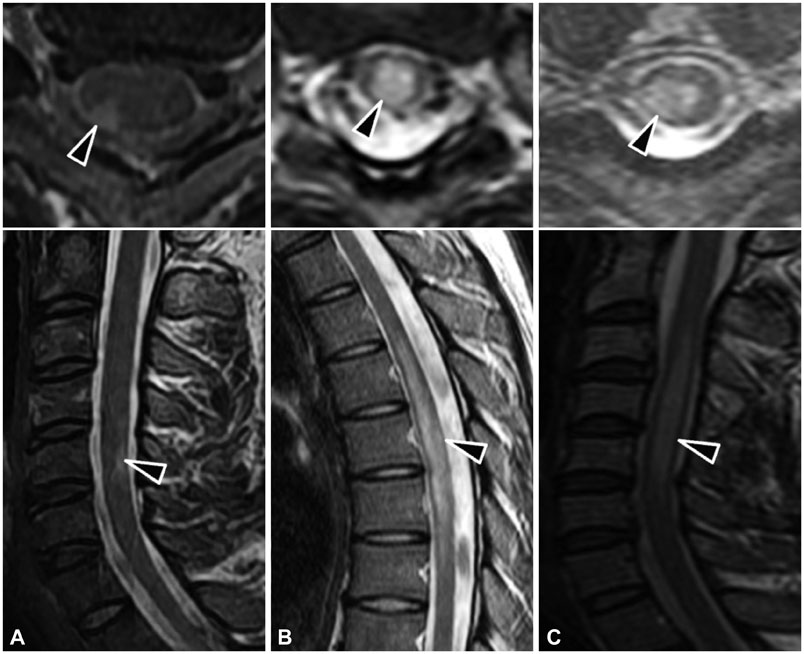
Of a total of 341 acute myelitis patients, 52 (15%) were identified as having acute sensory myelitis. The male-to-female ratio of these patients was 35:17, and their age at the onset of the condition was 41.7+/-10.5 years (mean+/-SD; range, 24-72 years). Acute sensory myelitis developed in patients with multiple sclerosis (MS; 14%), neuromyelitis optica spectrum disorder (NMOSD; 17%), and acute myelitis associated with concurrent systemic diseases including Behcet's disease and cancer (6%). Despite detailed evaluation, the etiology of 33 patients with acute myelitis could not be determined. Longitudinally extensive transverse myelitis on spinal MRI and progression of the sensory level were observed most commonly in NMOSD patients (89% and 78%, respectively); however, these patients did not exhibit sensory dissociation. Residual negative sensory symptoms were observed more frequently in NMOSD patients (33%) than in those with acute myelitis of unknown cause (24%) or MS (14%). During the long-term follow-up (4.7+/-2.7 years) of patients who did not undergo maintenance immunotherapy, a monophasic clinical course was common in those with acute myelitis of unknown cause (76%), but not in NMOSD or MS patients.
CONCLUSIONS
Accurate identification of the diverse nature of acute sensory myelitis may assist in patient care.
MeSH Terms
Figure
Reference
-
1. Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002; 59:499–505.2. Frohman EM, Wingerchuk DM. Clinical practice. Transverse myelitis. N Engl J Med. 2010; 363:564–572.3. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007; 6:805–815.
Article4. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol. 2005; 58:840–846.
Article5. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011; 69:292–302.
Article6. Jarius S, Probst C, Borowski K, Franciotta D, Wildemann B, Stoecker W, et al. Standardized method for the detection of antibodies to aquaporin-4 based on a highly sensitive immunofluorescence assay employing recombinant target antigen. J Neurol Sci. 2010; 291:52–56.
Article7. Sato DK, Nakashima I, Takahashi T, Misu T, Waters P, Kuroda H, et al. Aquaporin-4 antibody-positive cases beyond current diagnostic criteria for NMO spectrum disorders. Neurology. 2013; 80:2210–2216.
Article8. Kim W, Lee JE, Li XF, Kim SH, Han BG, Lee BI, et al. Quantitative measurement of anti-aquaporin-4 antibodies by enzyme-linked immunosorbent assay using purified recombinant human aquaporin-4. Mult Scler. 2012; 18:578–586.
Article9. Freedman MS, Thompson EJ, Deisenhammer F, Giovannoni G, Grimsley G, Keir G, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol. 2005; 62:865–870.10. Pittock SJ, Lucchinetti CF, Parisi JE, Benarroch EE, Mokri B, Stephan CL, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. 2005; 58:96–107.
Article11. Kira J, Yamasaki K, Kawano Y, Kobayashi T. Acute myelitis associated with hyperIgEemia and atopic dermatitis. J Neurol Sci. 1997; 148:199–203.
Article12. Yoon JH, Joo IS, Li WY, Sohn SY. Clinical and laboratory characteristics of atopic myelitis: Korean experience. J Neurol Sci. 2009; 285:154–158.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concomitant Acute Transverse Myelitis and Sensory Motor Axonal Polyneuropathy in Two Children: Two Case Reports
- A Case of Acute Transverse Myelitis with Hepatitis B Virus Infection
- Neuromyelitis Optica Spectrum Disorder Presented with Acute Memory Loss
- Acute Myelitis after hepatitis B vaccination
- Acute transverse myelitis caused by Coxsackie virus B4 infection: a case report