Anat Cell Biol.
2014 Sep;47(3):171-179. 10.5115/acb.2014.47.3.171.
Effect of saffron (Crocus sativus L.) on sodium valporate induced cytogenetic and testicular alterations in albino rats
- Affiliations
-
- 1Department of Zoology, Faculty of Science, Menoufia University, Menoufia, Egypt. sabsak@yahoo.com
- 2Department of Zoology, Faculty of Science, Benha University, Qalyubia, Egypt.
- KMID: 1882592
- DOI: http://doi.org/10.5115/acb.2014.47.3.171
Abstract
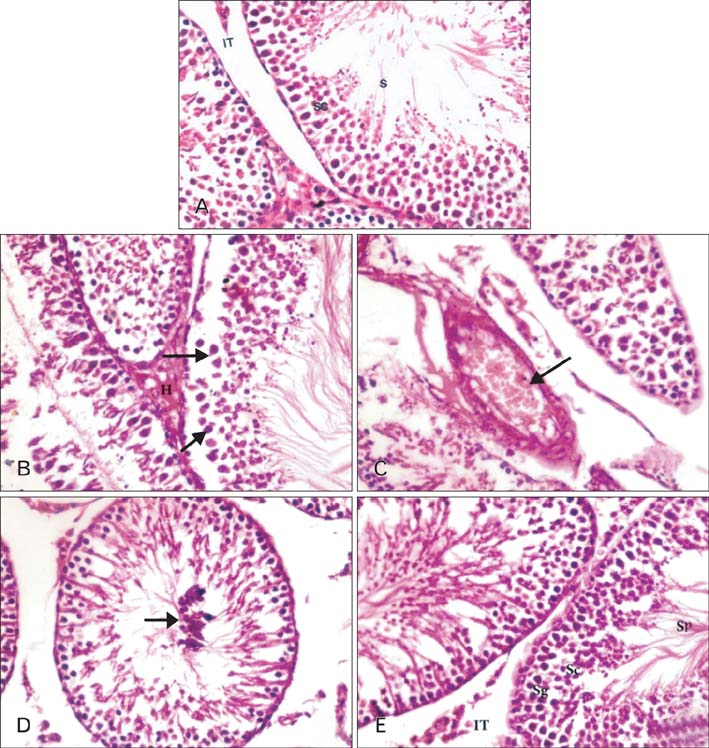
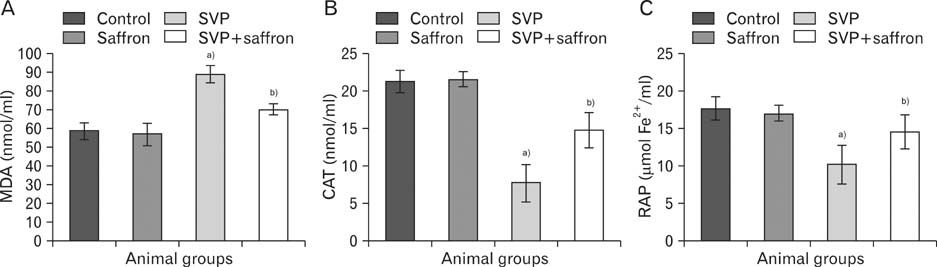
- The present study investigated the cytogenetic and testicular damage induced by the antiepileptic drug, sodium valporate (SVP) in albino rats and the effect of saffron aqueous extracts. Treating rats with SVP caused a significant increase in the chromosomal aberrations either structural or numerical and decreased the mitotic index. Besides, animals administered SVP showed DNA damage appeared in the single strand breaks (comet assay). Testis of SVP-treated rats showed many histopathological changes. A significant decrease in seminiferous tubules and their epithelial heights diameters and inhibition of spermatogenesis was recorded. In addition, the number of sperm head abnormalities was increased. Biochemical results revealed an increase in malondialdhyde (MDA) which is lipid peroxidation marker and a significant decrease in the level of serum antioxidant enzyme, catalase (CAT) and reducing antioxidant power (RAP). Animals given SVP and saffron showed an improvement in chromosomal aberrations, mitotic index, DNA damage and testicular alterations caused by SVP. Moreover, MDA decreased and CAT and RAP increased. It is concluded from the present results that the ameliorative effects of saffron extract against SVP-induced cytogenetic and testicular damage in albino rats may be due to the presence of one or more antioxidant components of saffron.
Keyword
MeSH Terms
Figure
Reference
-
1. Gelder M, Mayou R, Geddes J. Psychiatry. 3rd ed. Oxford: Oxford University Press;2006.2. Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002; 16:669–694.3. Kingsley E, Gray P, Tolman KG, Tweedale R. The toxicity of metabolites of sodium valproate in cultured hepatocytes. J Clin Pharmacol. 1983; 23:178–185.4. Witczak M, Kociszewska I, Wilczyński J, Lopaczyńska D, Ferenc T. Evaluation of chromosome aberrations, sister chromatid exchange and micronuclei in cultured cord-blood lymphocytes of newborns of women treated for epilepsy during pregnancy. Mutat Res. 2010; 701:111–117.5. Jentink J, Loane MA, Dolk H, Barisic I, Garne E, Morris JK, de Jong-van den Berg LT. EUROCAT Antiepileptic Study Working Group. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010; 362:2185–2193.6. Isojarvi JI, Lofgren E, Juntunen KS, Pakarinen AJ, Päivänsalo M, Rautakorpi I, Tuomivaara L. Effect of epilepsy and antiepileptic drugs on male reproductive health. Neurology. 2004; 62:247–253.7. Bairy L, Paul V, Rao Y. Reproductive toxicity of sodium valproate in male rats. Indian J Pharmacol. 2010; 42:90–94.8. Watkins JR, Gough AW, McGuire EJ, Goldenthal E, de la Iglesia FA. Calcium valproate-induced uterine adenocarcinomas in Wistar rats. Toxicology. 1992; 71:35–47.9. Vahidi H, Kamalinejad M, Sedaghati N. Antimicrobial properties of Crocus sativus L. Iran J Pharm Res. 2002; 1:33–35.10. Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigmas in mice. Arch Iran Med. 2002; 5:44–47.11. Hosseinzadeh H, Karimi G, Niapoor M. Antidepressant effects of Crocus sativus L. stigma extracts and their constituents, crocin and safranal, in mice. Acta Hortic. 2004; 650:435–445.12. Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002; 2:7.13. Nair SC, Pannikar B, Panikkar KR. Antitumour activity of saffron (Crocus sativus). Cancer Lett. 1991; 57:109–114.14. Premkumar K, Abraham SK, Santhiya ST, Ramesh A. Protective effects of saffron (Crocus sativus Linn.) on genotoxins-induced oxidative stress in Swiss albino mice. Phytother Res. 2003; 17:614–617.15. Iranshahi M, Khoshangosht M, Mohammadkhani Z, Karimi G. Protective effects of aqueous and ethanolic extracts of saffron stigma and petal on liver toxicity induced by carbon tetrachloride in mice. Pharmacologyonline. 2011; 1:203–212.16. Naghizadeh B, Boroushaki MT, Vahdati Mashhadian N, Mansouri MT. Protective effects of crocin against cisplatin-induced acute renal failure and oxidative stress in rats. Iran Biomed J. 2008; 12:93–100.17. Paget GE, Barnes JM. Toxicity tests. In : Laurence DR, Bacharach AL, editors. Evaluation of Drug Activities Pharmacometries. London and New York: Academic Press;1964. p. 135.18. Kashiwada E, Kuroda K, Endo G. Aneuploidy induced by dimethylarsinic acid in mouse bone marrow cells. Mutat Res. 1998; 413:33–38.19. Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988; 175:184–191.20. Wyrobek AJ, Bruce WR. Chemical induction of sperm abnormalities in mice. Proc Natl Acad Sci U S A. 1975; 72:4425–4429.21. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.22. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996; 239:70–76.23. Aebi H, Wyss SR, Scherz B, Skvaril F. Heterogeneity of erythrocyte catalase II. Isolation and characterization of normal and variant erythrocyte catalase and their subunits. Eur J Biochem. 1974; 48:137–145.24. Hu LJ, Lu XF, Lu BQ, Huang YQ. The effect of valproic acid on SCE and chromosome aberrations in epileptic children. Mutat Res. 1990; 243:63–66.25. Karapidaki I, Ekonomopoulou MT, Akritopoulou K, Anestakis D, Iakovidou-Kritsi Z. Cytogenetic effects of valproic acid and ziprasidone in human lymphocyte cultures. Neuropsychobiology. 2011; 64:219–223.26. Denli M, Aydin HI, Döndaröz R, Özişik T, Erdem E, Baltaci V. Genotoxicity evaluation in female patients on valproic acid monotherapy using alkaline single cell gel electrophoresis (comet assay). East J Med. 2000; 5:61–65.27. Felisbino MB, Tamashiro WM, Mello ML. Chromatin remodeling, cell proliferation and cell death in valproic acid-treated HeLa cells. PLoS One. 2011; 6:e29144.28. Li Y, Liu T, Ivan C, Huang J, Shen DY, Kavanagh JJ, Bast RC Jr, Fu S, Hu W, Sood AK. Enhanced cytotoxic effects of combined valproic acid and the aurora kinase inhibitor VE465 on gynecologic cancer cells. Front Oncol. 2013; 3:58.29. Khan S, Ahmad T, Parekh CV, Trivedi PP, Kushwaha S, Jena G. Investigation on sodium valproate induced germ cell damage, oxidative stress and genotoxicity in male Swiss mice. Reprod Toxicol. 2011; 32:385–394.30. Roste LS, Taubøll E, Haugen TB, Bjornenak T, Saetre ER, Gjerstad L. Alterations in semen parameters in men with epilepsy treated with valproate or carbamazepine monotherapy. Eur J Neurol. 2003; 10:501–506.31. Cansu A, Ekinci O, Serdaroglu A, Gürgen SG, Ekinci O, Erdogan D, Coskun ZK, Tunc L. Effects of chronic treatment with valproate and oxcarbazepine on testicular development in rats. Seizure. 2011; 20:203–207.32. Vidya M, Subramanian S. Effects of micro-ketoglutarate on antioxidants and lipid peroxidation products in rats treated with sodium valproate. J Appl Biomed. 2006; 4:141–146.33. Sobaniec W, Solowiej E, Kulak W, Bockowski L, Smigielska-Kuzia J, Artemowicz B. Evaluation of the influence of antiepileptic therapy on antioxidant enzyme activity and lipid peroxidation in erythrocytes of children with epilepsy. J Child Neurol. 2006; 21:558–562.34. Zhang YJ, Zhang M, Wang XC, Yu YH, Jin PJ, Wang Y. Effects of sodium valproate on neutrophils\' oxidative metabolism and oxidant status in children with idiopathic epilepsy. Zhonghua Er Ke Za Zhi. 2011; 49:776–781.35. Hosseinzadeh H, Sadeghnia HR. Effect of safranal, a constituent of Crocus sativus (saffron), on methyl methanesulfonate (MMS)-induced DNA damage in mouse organs: an alkaline single-cell gel electrophoresis (comet) assay. DNA Cell Biol. 2007; 26:841–846.36. Asadi MH, Zafari F, Sarveazad A, Abbasi M, Safa M, Koruji M, Yari A, Alizadeh Miran R. Saffron improves epididymal sperm parameters in rats exposed to cadmium. Nephrourol Mon. 2014; 6:e12125.37. Heidary M, Vahhabi S, Reza Nejadi J, Delfan B, Birjandi M, Kaviani H, Givrad S. Effect of saffron on semen parameters of infertile men. Urol J. 2008; 5:255–259.38. Modaresi M, Mesripour M, Asadi Margh, Hamedanian MK. Effect of Saffron (Crocus sativus) extract on level of FSH, LH and testosterone in mice. J Zanjan Univ Med Sci Health Serv. 2008; 16:11–17.39. Goli SA, Mokhtari F, Rahimmalek M. Phenolic compounds and antioxidant activity from Saffron (Crocus sativus L.) Petal. J Agric Sci. 2012; 4:175–180.40. Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituent, crocin and safranal. Pharmacogn Mag. 2009; 5:419–424.41. Shati AA, Alamri SA. Role of saffron (Crocus sativus L.) and honey syrup on aluminum-induced hepatotoxicity. Saudi Med J. 2010; 31:1106–1113.42. Vakili A, Einali MR, Bandegi AR. Protective effect of crocin against cerebral ischemia in a dose-dependent manner in a rat model of ischemic stroke. J Stroke Cerebrovasc Dis. 2014; 23:106–113.43. Mohajeri D, Doustar Y. Protective effect of ethanolic extract of Crocus sativus L. (saffron) stigma against cisplatin induced hepatotoxicity in rats. Med Sci J Islamic Azad Univ. 2012; 21:251–261.44. Giaccio M. Crocetin from saffron: an active component of an ancient spice. Crit Rev Food Sci Nutr. 2004; 44:155–172.45. Papandreou MA, Tsachaki M, Efthimiopoulos S, Cordopatis P, Lamari FN, Margarity M. Memory enhancing effects of saffron in aged mice are correlated with antioxidant protection. Behav Brain Res. 2011; 219:197–204.46. Rios JL, Recio MC, Giner RM, Máñez S. An update review of saffron and its active constituents. Phytother Res. 1996; 10:189–193.47. Assimopoulou AN, Sinakos Z, Papageorgiou VP. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005; 19:997–1000.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of a Saffron Extract (affron ® ) on Menopausal Symptoms in Women during Perimenopause: A Randomised, Double-Blind, Placebo-Controlled Study
- Protective effects of saffron against zearalenone-induced alterations in reproductive hormones in female mice (Mus musculus)
- Effect of Ocimum basilicum extract on cadmium-induced testicular histomorphometric and immunohistochemical alterations in albino rats
- The effect of testicular torsion on the contralateral testis in rats
- Protective effect of diallyl disulfide on cyclophosphamide-induced testicular toxicity in rats