Anat Cell Biol.
2014 Jun;47(2):101-110. 10.5115/acb.2014.47.2.101.
Region-specific changes in the immunoreactivity of Atg9A in the central nervous system of SOD1(G93A) transgenic mice
- Affiliations
-
- 1Department of Anatomy, Seoul National University College of Medicine, Seoul, Korea. cicha@snu.ac.kr
- 2Department of Biology, School of Life Sciences, Chungbuk National University, Cheongju, Korea. schoe@chungbuk.ac.kr
- KMID: 1882580
- DOI: http://doi.org/10.5115/acb.2014.47.2.101
Abstract
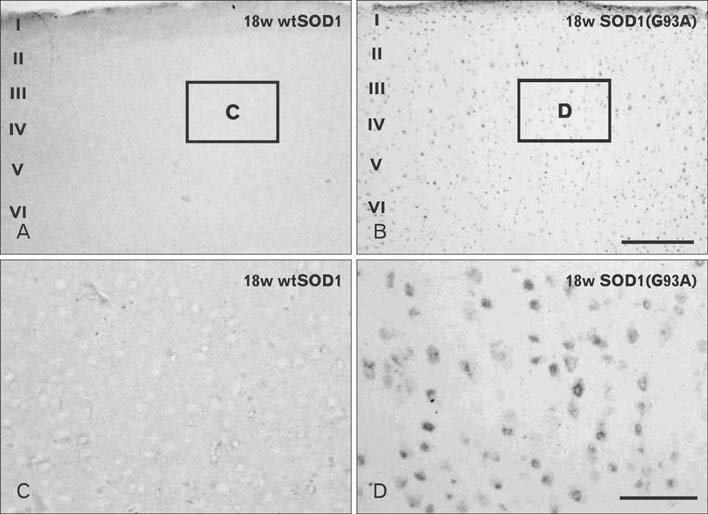
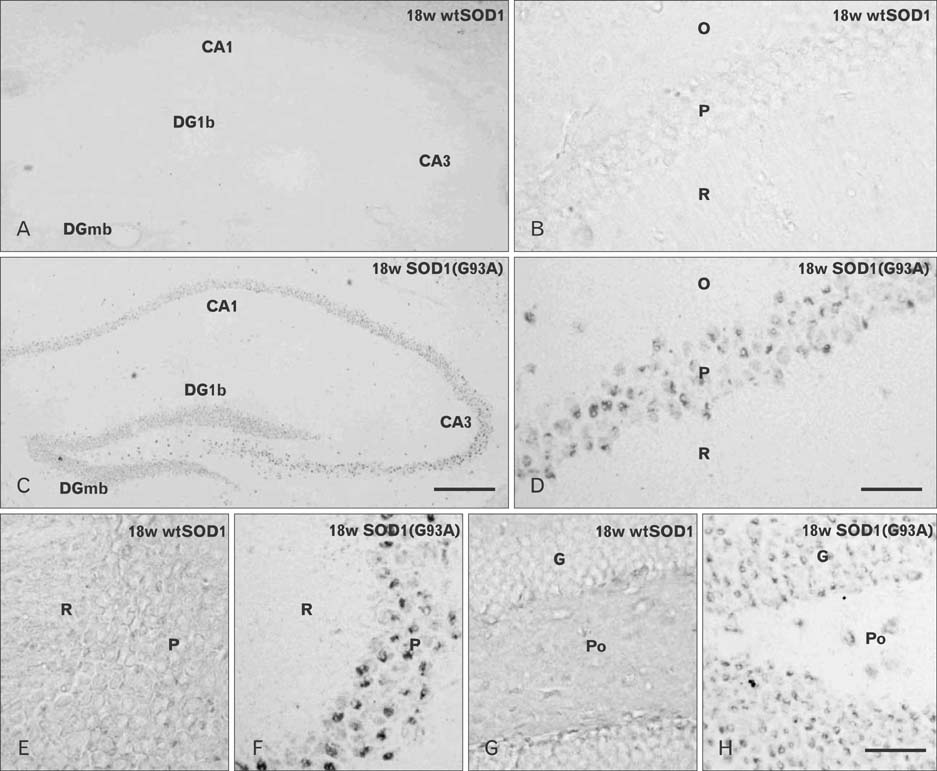
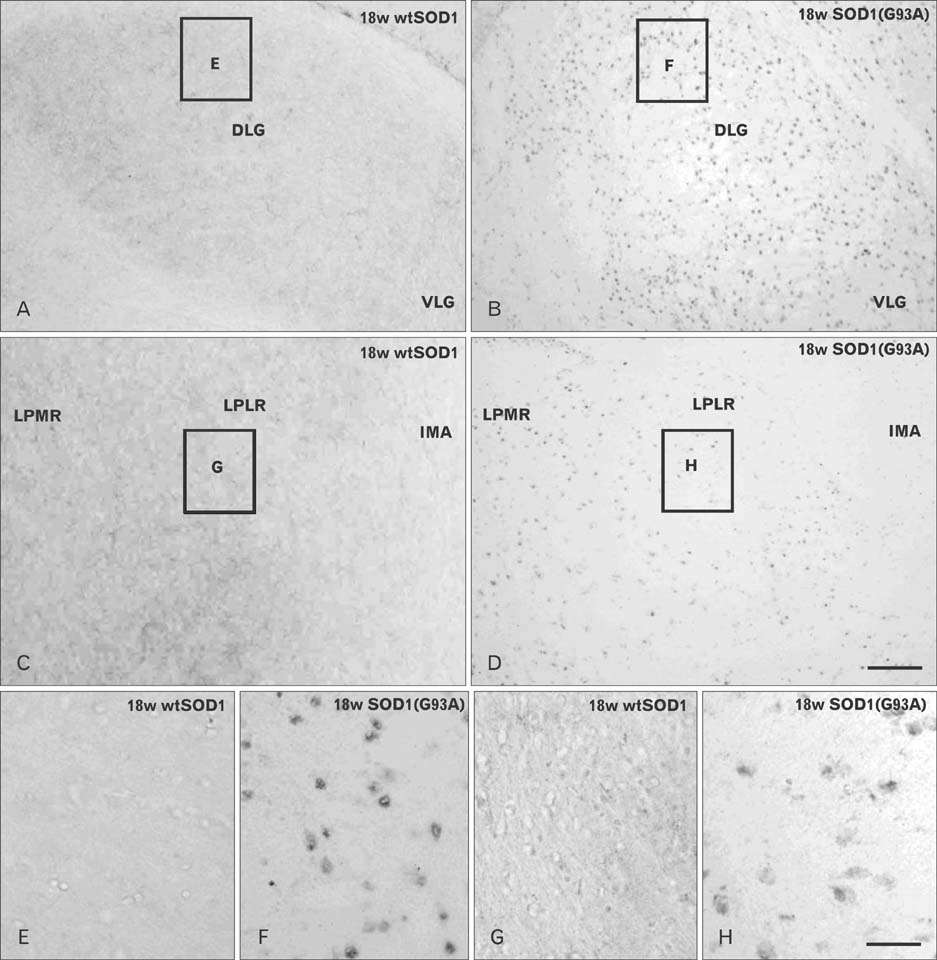
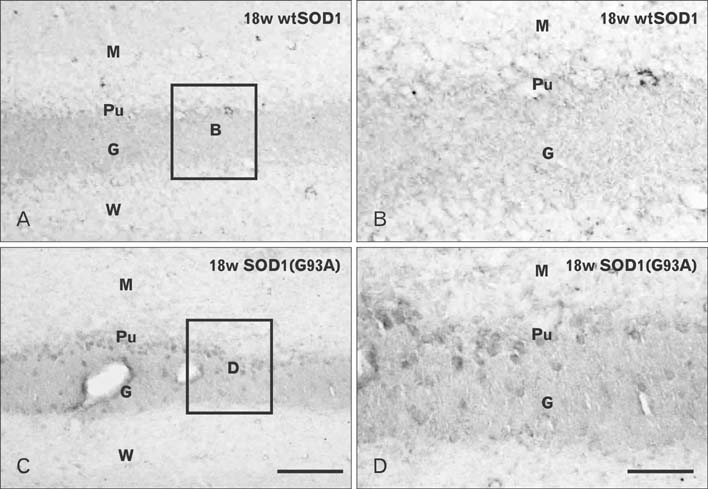
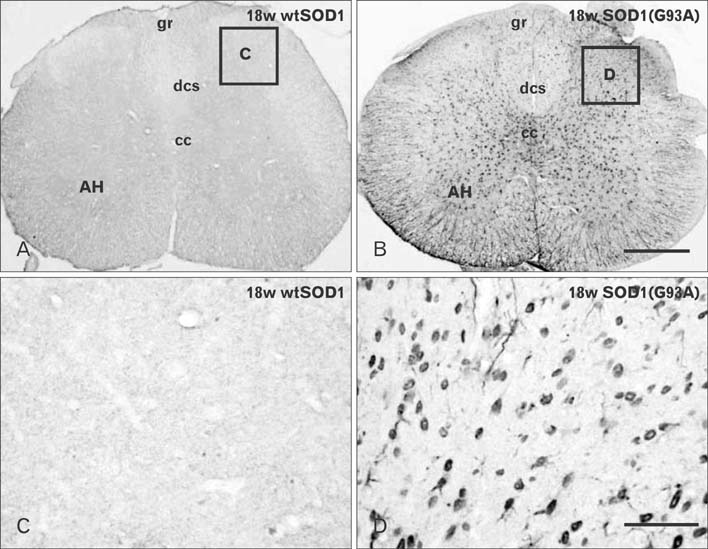
- Autophagy is a eukaryotic self-degradation system that plays a pivotal role in the maintenance of cellular homeostasis. Atg9 is the only transmembrane Atg protein required for autophagosome formation. Although the subcellular localization of the Atg9A has been examined, little is known about its precise cell and tissue distribution. In the present study, we used G93A mutation in superoxide dismutase 1 [SOD1(G93A)] mutant transgenic mice as an in vivo model of amyotrophic lateral sclerosis (ALS) and performed immunohistochemical studies to investigate the changes of Atg9A immunoreactivity in the central nervous system of these mice. Atg9A-immunoreactivity was detected in the spinal cord, cerebral cortex, hippocampal formation, thalamus and cerebellum of symptomatic SOD1(G93A) transgenic mice. By contrast, no Atg9A-immunoreactivity were observed in any brain and spinal cord region of wtSOD1, pre-symptomatic and early symptomatic mice, and the number and staining intensity of Atg9A-positive cells did not differ in SOD1(G93A) mice between 8 and 13 weeks of age. These results provide evidence that Atg9A-immunoreactivity were found in the central nervous system of SOD1(G93A) transgenic mice after clinical symptoms, suggesting a possible role in the pathologic process of ALS. However, the mechanisms underlying the increased immunoreactivity for Atg9A and the functional implications require elucidation.
Keyword
MeSH Terms
Figure
Reference
-
1. Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001; 2:806–819.2. Venerosi A, Martire A, Rungi A, Pieri M, Ferrante A, Zona C, Popoli P, Calamandrei G. Complex behavioral and synaptic effects of dietary branched chain amino acids in a mouse model of amyotrophic lateral sclerosis. Mol Nutr Food Res. 2011; 55:541–552.3. Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998; 281:1851–1854.4. Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994; 264:1772–1775.5. Nagai M, Aoki M, Miyoshi I, Kato M, Pasinelli P, Kasai N, Brown RH Jr, Itoyama Y. Rats expressing human cytosolic copper-zinc superoxide dismutase transgenes with amyotrophic lateral sclerosis: associated mutations develop motor neuron disease. J Neurosci. 2001; 21:9246–9254.6. Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Rahmani Z, Krizus A, Mckenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, van den Bergh R, Hung WY, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH Jr. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993; 362:59–62.7. Murphy J, Henry R, Lomen-Hoerth C. Establishing subtypes of the continuum of frontal lobe impairment in amyotrophic lateral sclerosis. Arch Neurol. 2007; 64:330–334.8. Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007; 6:994–1003.9. Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005; 65:586–590.10. Klionsky DJ, Cregg JM, Dunn WA Jr, Emr SD, Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M, Ohsumi Y. A unified nomenclature for yeast autophagy-related genes. Dev Cell. 2003; 5:539–545.11. Tamura H, Shibata M, Koike M, Sasaki M, Uchiyama Y. Atg9A protein, an autophagy-related membrane protein, is localized in the neurons of mouse brains. J Histochem Cytochem. 2010; 58:443–453.12. Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005; 12:Suppl 2. 1542–1552.13. Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004; 6:463–477.14. Reggiori F, Klionsky DJ. Autophagy in the eukaryotic cell. Eukaryot Cell. 2002; 1:11–21.15. Kirisako T, Baba M, Ishihara N, Miyazawa K, Ohsumi M, Yoshimori T, Noda T, Ohsumi Y. Formation process of autophagosome is traced with Apg8/Aut7p in yeast. J Cell Biol. 1999; 147:435–446.16. Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006; 441:885–889.17. Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006; 441:880–884.18. Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006; 443:780–786.19. Krick R, Muehe Y, Prick T, Bremer S, Schlotterhose P, Eskelinen EL, Millen J, Goldfarb DS, Thumm M. Piecemeal microautophagy of the nucleus requires the core macroautophagy genes. Mol Biol Cell. 2008; 19:4492–4505.20. Sekito T, Kawamata T, Ichikawa R, Suzuki K, Ohsumi Y. Atg17 recruits Atg9 to organize the pre-autophagosomal structure. Genes Cells. 2009; 14:525–538.21. Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001; 20:5971–5981.22. Yamada T, Carson AR, Caniggia I, Umebayashi K, Yoshimori T, Nakabayashi K, Scherer SW. Endothelial nitric-oxide synthase antisense (NOS3AS) gene encodes an autophagy-related protein (APG9-like2) highly expressed in trophoblast. J Biol Chem. 2005; 280:18283–18290.23. Young AR, Chan EY, Hu XW, Köchl R, Crawshaw SG, High S, Hailey DW, Lippincott-Schwartz J, Tooze SA. Starvation and ULK1-dependent cycling of mammalian Atg9 between the TGN and endosomes. J Cell Sci. 2006; 119(Pt 18):3888–3900.24. Lee JC, Chung YH, Cho YJ, Kim J, Kim N, Cha CI, Joo KM. Immunohistochemical study on the expression of calcium binding proteins (calbindin-D28k, calretinin, and parvalbumin) in the cerebellum of the nNOS knock-out(-/-) mice. Anat Cell Biol. 2010; 43:64–71.25. Lee JC, Shin JH, Park BW, Kim GS, Kim JC, Kang KS, Cha CI. Region-specific changes in the immunoreactivity of SIRT1 expression in the central nervous system of SOD1(G93A) transgenic mice as an in vivo model of amyotrophic lateral sclerosis. Brain Res. 2012; 1433:20–28.26. Webber JL, Young AR, Tooze SA. Atg9 trafficking in mammalian cells. Autophagy. 2007; 3:54–56.27. Aguib Y, Heiseke A, Gilch S, Riemer C, Baier M, Schätzl HM, Ertmer A. Autophagy induction by trehalose counteracts cellular prion infection. Autophagy. 2009; 5:361–369.28. Fornai F, Longone P, Cafaro L, Kastsiuchenka O, Ferrucci M, Manca ML, Lazzeri G, Spalloni A, Bellio N, Lenzi P, Modugno N, Siciliano G, Isidoro C, Murri L, Ruggieri S, Paparelli A. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2008; 105:2052–2057.29. Sasaki S. Autophagy in spinal cord motor neurons in sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2011; 70:349–359.30. Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC. Alpha-Synuclein is degraded by both autophagy and the proteasome. J Biol Chem. 2003; 278:25009–25013.31. Li L, Zhang X, Le W. Altered macroautophagy in the spinal cord of SOD1 mutant mice. Autophagy. 2008; 4:290–293.32. Morimoto N, Nagai M, Ohta Y, Miyazaki K, Kurata T, Morimoto M, Murakami T, Takehisa Y, Ikeda Y, Kamiya T, Abe K. Increased autophagy in transgenic mice with a G93A mutant SOD1 gene. Brain Res. 2007; 1167:112–117.33. Rusten TE, Simonsen A. ESCRT functions in autophagy and associated disease. Cell Cycle. 2008; 7:1166–1172.34. Agosta F, Pagani E, Rocca MA, Caputo D, Perini M, Salvi F, Prelle A, Filippi M. Voxel-based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability. Hum Brain Mapp. 2007; 28:1430–1438.35. Kew JJ, Goldstein LH, Leigh PN, Abrahams S, Cosgrave N, Passingham RE, Frackowiak RS, Brooks DJ. The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis. A neuropsychological and positron emission tomography study. Brain. 1993; 116(Pt 6):1399–1423.36. Rose S, Pannek K, Bell C, Baumann F, Hutchinson N, Coulthard A, McCombe P, Henderson R. Direct evidence of intra- and interhemispheric corticomotor network degeneration in amyotrophic lateral sclerosis: an automated MRI structural connectivity study. Neuroimage. 2012; 59:2661–2669.37. Vercelletto M, Ronin M, Huvet M, Magne C, Feve JR. Frontal type dementia preceding amyotrophic lateral sclerosis: a neuropsychological and SPECT study of five clinical cases. Eur J Neurol. 1999; 6:295–299.38. Nagata T, Ilieva H, Murakami T, Shiote M, Narai H, Ohta Y, Hayashi T, Shoji M, Abe K. Increased ER stress during motor neuron degeneration in a transgenic mouse model of amyotrophic lateral sclerosis. Neurol Res. 2007; 29:767–771.39. Sasaki S. Endoplasmic reticulum stress in motor neurons of the spinal cord in sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2010; 69:346–355.40. Mizushima N. Autophagy: process and function. Genes Dev. 2007; 21:2861–2873.41. Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010; 140:313–326.42. Zhou C, Zhao CP, Zhang C, Wu GY, Xiong F, Zhang C. A method comparison in monitoring disease progression of G93A mouse model of ALS. Amyotroph Lateral Scler. 2007; 8:366–372.43. Song CY, Guo JF, Liu Y, Tang BS. Autophagy and Its Comprehensive Impact on ALS. Int J Neurosci. 2012; 122:695–703.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rosmarinic Acid Alleviates Neurological Symptoms in the G93A-SOD1 Transgenic Mouse Model of Amyotrophic Lateral Sclerosis
- Modulation of SOD1 Subcellular Localization by Transfection with Wild- or Mutant-type SOD1 in Primary Neuron and Astrocyte Cultures from ALS Mice
- Immunohistochemical Study on the Distribution of Glycogen Synthase Kinase (GSK) 3beta in the Central Nervous System of SOD1G93A Transgenic Mice
- Sensory involvement in the SOD1-G93A mouse model of amyotrophic lateral sclerosis
- Neuroprotective Effect of Rapamycin (Autophagy Enhancer) in Transgenic SOD1-G93A Mice of Amyotrophic Lateral Sclerosis