Anat Cell Biol.
2014 Jun;47(2):91-100. 10.5115/acb.2014.47.2.91.
FK506 reduces calpain-regulated calcineurin activity in both the cytoplasm and the nucleus
- Affiliations
-
- 1Department of Anatomy and Neurobiology, Institute of Health Sciences, Medical Research Center for Neural Dysfunction, Gyeongsang National University School of Medicine, Jinju, Korea. kjcho@gnu.ac.kr
- KMID: 1882579
- DOI: http://doi.org/10.5115/acb.2014.47.2.91
Abstract
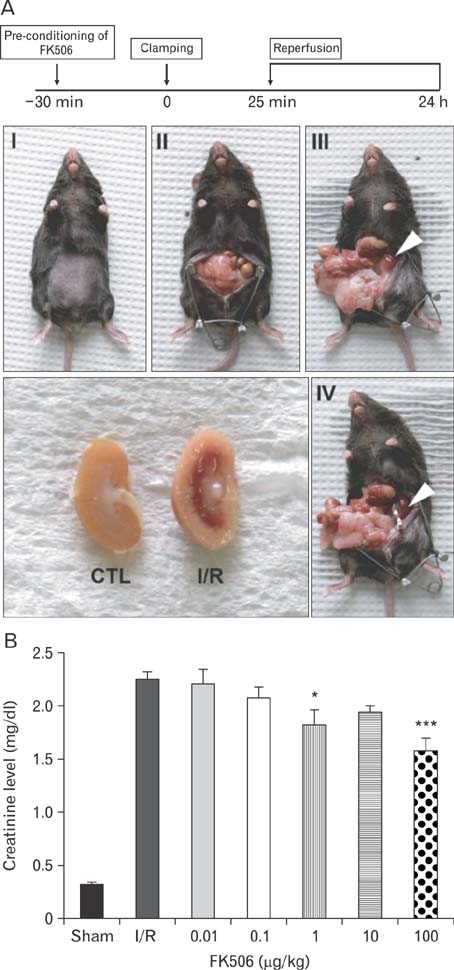
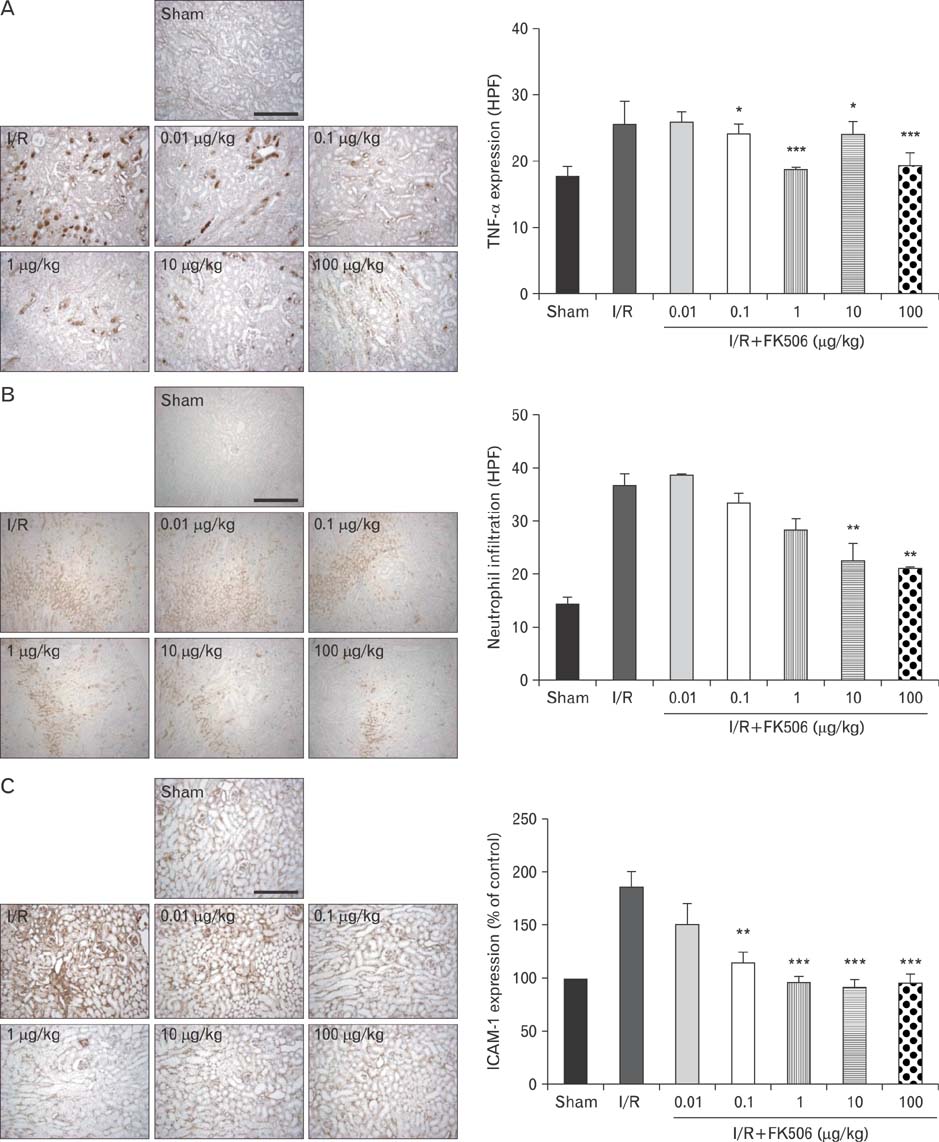
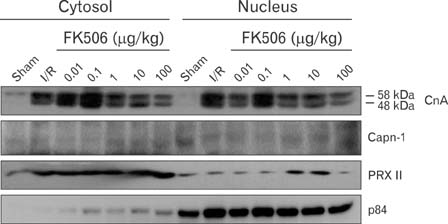
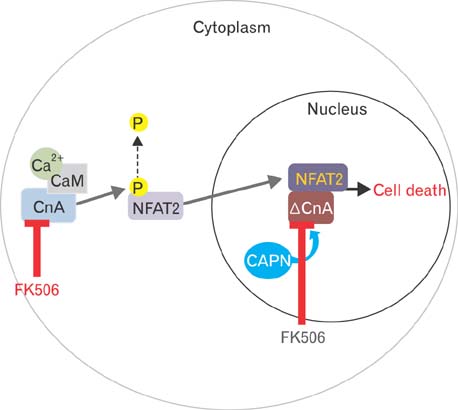
- Excessive immune responses induced by ischemia-reperfusion injury (IRI) are known to lead to necrotic and apoptotic cell death, and calcineurin plays a major role in this process. Calcineurin dephosphorylates the nuclear factor of activated T-cells (NFAT), permitting its translocation into the nucleus. As a result, calcineurin promotes the release of pro-inflammatory cytokines, such as tumor necrosis factor-alpha. The overproduction of pro-inflammatory cytokines causes renal cell death. Calcineurin activity is regulated by calpain, a cysteine protease present in the nucleus. Calpain-mediated proteolysis increases the phosphatase activity of calcineurin, resulting in NFAT dephosphorylation. This process has been studied in cardiomyocytes but its role in renal IRI is unknown. Thus, we examined whether calpain regulates calcineurin in renal tubule nuclei. We established an in vivo renal IRI model in mice and identified the protective role of a calcineurin inhibitor, FK506, in this process. Calcineurin is expressed in the nucleus, where it is present in its calpain-cleaved form. FK506 reduced nuclear expression of calcineurin and prevented calcineurin-mediated NFAT activation. Our study shows clearly that FK506 reduces calpain-mediated calcineurin activity. Consequently, calcineurin could not maintain NFAT activation. FK506 reduced renal cell death by suppressing the transcription of pro-inflammatory cytokine genes. This study provides evidence that FK506 protects against inflammation in a renal IRI mouse model. We also provided a mechanism of calcineurin action in the nucleus. Therefore, FK506 could improve renal function by decreasing calcineurin activity in both the cytoplasm and the nucleus of renal tubule cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Neudoerfl C, Mueller BJ, Blume C, Daemen K, Stevanovic-Meyer M, Keil J, Lehner F, Haller H, Falk CS. The peripheral NK cell repertoire after kidney transplantation is modulated by different immunosuppressive drugs. Front Immunol. 2013; 4:46.2. Zhou W, Guan Q, Kwan CC, Chen H, Gleave ME, Nguan CY, Du C. Loss of clusterin expression worsens renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2010; 298:F568–F578.3. Shihab FS, Bennett WM, Andoh TF. Donor preconditioning with a calcineurin inhibitor improves outcome in rat syngeneic kidney transplantation. Transplantation. 2009; 87:326–329.4. Kennedy SE, Erlich JH. Murine renal ischaemia-reperfusion injury. Nephrology (Carlton). 2008; 13:390–396.5. Akool el-S, Doller A, Babelova A, Tsalastra W, Moreth K, Schaefer L, Pfeilschifter J, Eberhardt W. Molecular mechanisms of TGF beta receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporin A and FK506. J Immunol. 2008; 181:2831–2845.6. Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol. 2003; 284:F144–F154.7. Gooch JL, Pèrgola PE, Guler RL, Abboud HE, Barnes JL. Differential expression of calcineurin A isoforms in the diabetic kidney. J Am Soc Nephrol. 2004; 15:1421–1429.8. Heineke J, Ritter O. Cardiomyocyte calcineurin signaling in subcellular domains: from the sarcolemma to the nucleus and beyond. J Mol Cell Cardiol. 2012; 52:62–73.9. Kilka S, Erdmann F, Migdoll A, Fischer G, Weiwad M. The proline-rich N-terminal sequence of calcineurin Abeta determines substrate binding. Biochemistry. 2009; 48:1900–1910.10. Siepert A, Brosel S, Vogt K, Ahrlich S, Schmitt-Knosalla I, Loddenkemper C, Kühl A, Baumgrass R, Gerstmayer B, Tomiuk S, Tiedge M, Viklický O, Brabcova I, Nizze H, Lehmann M, Volk HD, Sawitzki B. Mechanisms and rescue strategies of calcineurin inhibitor mediated tolerance abrogation induced by anti-CD4 mAb treatment. Am J Transplant. 2013; 13:2308–2321.11. Gorentla BK, Zhong XP. T cell receptor signal transduction in T lymphocytes. J Clin Cell Immunol. 2012; 2012:Suppl 12. 5.12. Grigoriu S, Bond R, Cossio P, Chen JA, Ly N, Hummer G, Page R, Cyert MS, Peti W. The molecular mechanism of substrate engagement and immunosuppressant inhibition of calcineurin. PLoS Biol. 2013; 11:e1001492.13. Kurji K, Sharma RK. Potential role of calcineurin in pathogenic conditions. Mol Cell Biochem. 2010; 338:133–141.14. Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000; 80:1483–1521.15. Cook LG, Chiasson VL, Long C, Wu GY, Mitchell BM. Tacrolimus reduces nitric oxide synthase function by binding to FKBP rather than by its calcineurin effect. Kidney Int. 2009; 75:719–726.16. MacMillan D. FK506 binding proteins: cellular regulators of intracellular Ca2+ signalling. Eur J Pharmacol. 2013; 700:181–193.17. Burkard N, Becher J, Heindl C, Neyses L, Schuh K, Ritter O. Targeted proteolysis sustains calcineurin activation. Circulation. 2005; 111:1045–1053.18. Hisamitsu T, Nakamura TY, Wakabayashi S. Na(+)/H(+) exchanger 1 directly binds to calcineurin A and activates downstream NFAT signaling, leading to cardiomyocyte hypertrophy. Mol Cell Biol. 2012; 32:3265–3280.19. Liu G, Li M, Daneshgari F. Calcineurin and Akt expression in hypertrophied bladder in STZ-induced diabetic rat. Exp Mol Pathol. 2012; 92:210–216.20. Wilkins BJ, Molkentin JD. Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol. 2002; 541(Pt 1):1–8.21. Wu HY, Tomizawa K, Matsui H. Calpain-calcineurin signaling in the pathogenesis of calcium-dependent disorder. Acta Med Okayama. 2007; 61:123–137.22. Williams CR, Gooch JL. Calcineurin inhibitors and immunosuppression: a tale of two isoforms. Expert Rev Mol Med. 2012; 14:e14.23. Gooch JL. An emerging role for calcineurin Aalpha in the development and function of the kidney. Am J Physiol Renal Physiol. 2006; 290:F769–F776.24. Abdin AA. Targeting sphingosine kinase 1 (SphK1) and apoptosis by colon-specific delivery formula of resveratrol in treatment of experimental ulcerative colitis in rats. Eur J Pharmacol. 2013; 718:145–153.25. Feliers D, Gorin Y, Ghosh-Choudhury G, Abboud HE, Kasinath BS. Angiotensin II stimulation of VEGF mRNA translation requires production of reactive oxygen species. Am J Physiol Renal Physiol. 2006; 290:F927–F936.26. Minami T, Jiang S, Schadler K, Suehiro J, Osawa T, Oike Y, Miura M, Naito M, Kodama T, Ryeom S. The calcineurin-NFAT-angiopoietin-2 signaling axis in lung endothelium is critical for the establishment of lung metastases. Cell Rep. 2013; 4:709–723.27. Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004; 94:110–118.28. Xiao HB, Liu RH, Ling GH, Xiao L, Xia YC, Liu FY, Li J, Liu YH, Chen QK, Lv JL, Zhan M, Yang SK, Kanwar YS, Sun L. HSP47 regulates ECM accumulation in renal proximal tubular cells induced by TGF-beta1 through ERK1/2 and JNK MAPK pathways. Am J Physiol Renal Physiol. 2012; 303:F757–F765.29. Schofield ZV, Woodruff TM, Halai R, Wu MC, Cooper MA. Neutrophils: a key component of ischemia-reperfusion injury. Shock. 2013; 40:463–470.30. Qi XM, Wu YG, Liang C, Zhang P, Dong J, Ren KJ, Zhang W, Fang F, Shen JJ. FK506 ameliorates renal injury in early experimental diabetic rats induced by streptozotocin. Int Immunopharmacol. 2011; 11:1613–1619.31. Abdulkader RC, Liborio AB, Malheiros DM. Histological features of acute tubular necrosis in native kidneys and long-term renal function. Ren Fail. 2008; 30:667–673.32. Bueno OF, Lips DJ, Kaiser RA, Wilkins BJ, Dai YS, Glascock BJ, Klevitsky R, Hewett TE, Kimball TR, Aronow BJ, Doevendans PA, Molkentin JD. Calcineurin Abeta gene targeting predisposes the myocardium to acute ischemia-induced apoptosis and dysfunction. Circ Res. 2004; 94:91–99.33. Hashimoto Y, Soderling TR. Regulation of calcineurin by phosphorylation. Identification of the regulatory site phosphorylated by Ca2+/calmodulin-dependent protein kinase II and protein kinase C. J Biol Chem. 1989; 264:16524–16529.34. Park CH, Kim YS, Kim YH, Choi MY, Yoo JM, Kang SS, Choi WS, Cho GJ. Calcineurin mediates AKT dephosphorylation in the ischemic rat retina. Brain Res. 2008; 1234:148–157.35. Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999; 284:339–343.36. Yousuf S, Atif F, Kesherwani V, Agrawal SK. Neuroprotective effects of Tacrolimus (FK-506) and Cyclosporin (CsA) in oxidative injury. Brain Behav. 2011; 1:87–94.37. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med. 2006; 354:2473–2483.38. Stevens LA, Levey AS. Measurement of kidney function. Med Clin North Am. 2005; 89:457–473.39. Kashyap T, Rabinovitz I. The calcium/calcineurin pathway promotes hemidesmosome stability through inhibition of beta4 integrin phosphorylation. J Biol Chem. 2012; 287:32440–32449.40. Gooch JL, Gorin Y, Zhang BX, Abboud HE. Involvement of calcineurin in transforming growth factor-beta-mediated regulation of extracellular matrix accumulation. J Biol Chem. 2004; 279:15561–15570.41. Stefater JA 3rd, Rao S, Bezold K, Aplin AC, Nicosia RF, Pollard JW, Ferrara N, Lang RA. Macrophage Wnt-Calcineurin-Flt1 signaling regulates mouse wound angiogenesis and repair. Blood. 2013; 121:2574–2578.42. Zhang B, Shi W, Ma J, Sloan A, Faul C, Wei C, Reiser J, Yang Y, Liu S, Wang W. The calcineurin-NFAT pathway allows for urokinase receptor-mediated beta3 integrin signaling to cause podocyte injury. J Mol Med (Berl). 2012; 90:1407–1420.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MicroRNA profiling of tacrolimus-stimulated Jurkat human T lympocytes
- Apoptosis Gene Expression Pattern Analysis of Jurkat Cells Treated with FK506
- Calpain inhibitors reduce the cornified cell envelope formation by inhibiting proteolytic processing of transglutaminase 1
- Epidermal Cysts in a Tacrolimus Treated Renal Transplant Recipient
- Role of Calcineurin-Dependent Signaling Pathway on the Left Ventricular Hypertrophy Induced by Pressure Overload