J Lung Cancer.
2006 Jun;5(1):39-46. 10.6058/jlc.2006.5.1.39.
Heme Oxygenase-1 via Transcriptional Activation of Nrf2 Attenuates Apoptosis in Lung Cancer Cells
- Affiliations
-
- 1Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea. jetpul@wonkwang.ac.kr
- 2Department of Radiology, Wonkwang University School of Medicine, Iksan, Korea.
- KMID: 1881525
- DOI: http://doi.org/10.6058/jlc.2006.5.1.39
Abstract
-
PURPOSE : Increasing evidences have indicated the critical role of HO-1 in cytoprotection and more diverse biological functions. HO-1 has been reported to stimulate tumor cell growth and proliferation. And several tumors, including renal cell carcinoma, prostate tumor, hepatoma, and sarcoma, express a high level of HO-1. Indeed, inhibition of HO-1 by using specific HO inhibitors demonstrated in vivo antitumor activity. However, the precise mechanism of HO-1 induction and signals in lung cancer is not clearly known yet. We aimed to analyze the role of HO-1, characterize the mechanism of HO-1 induction, the role of Nrf2 in the induction, and investigated whether inhibition of HO-1 may induce apoptosis in lung cancer cells.
MATERIALS AND METHODS
: Western blot and immunostaining analyses were performed to ascertain whether HO-1, Nrf2, and NFkB were expressed or not in various lung cancer cell lines. Apoptosis by HO-1 inhibition through siRNA transfection was measured by flow cytometric analysis and Western blot. And the expression of HO-1 by siRNA of Nrf2 and NFkB was examined by ARE-driven luciferase activity and Western blot.
RESULTS
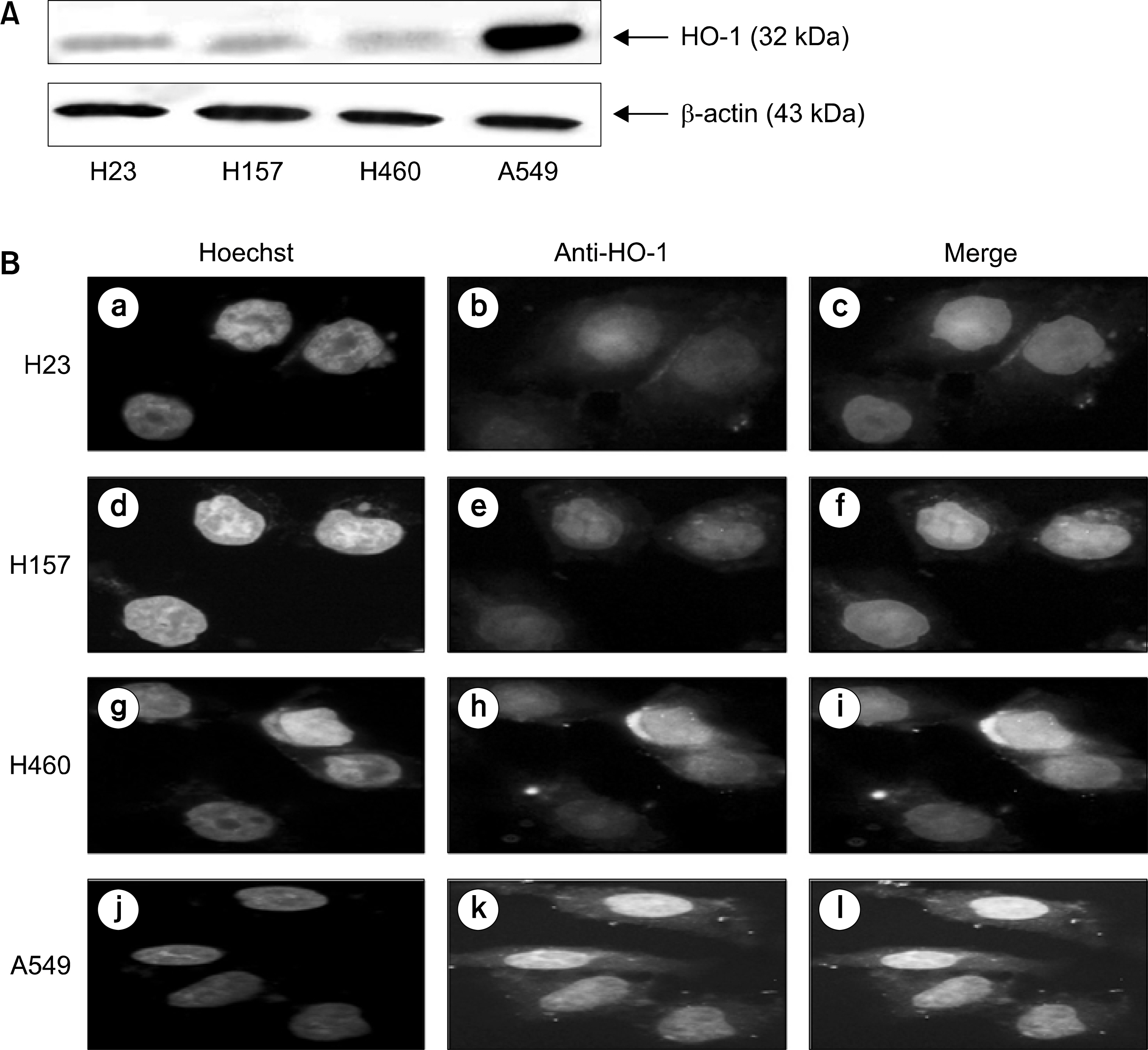
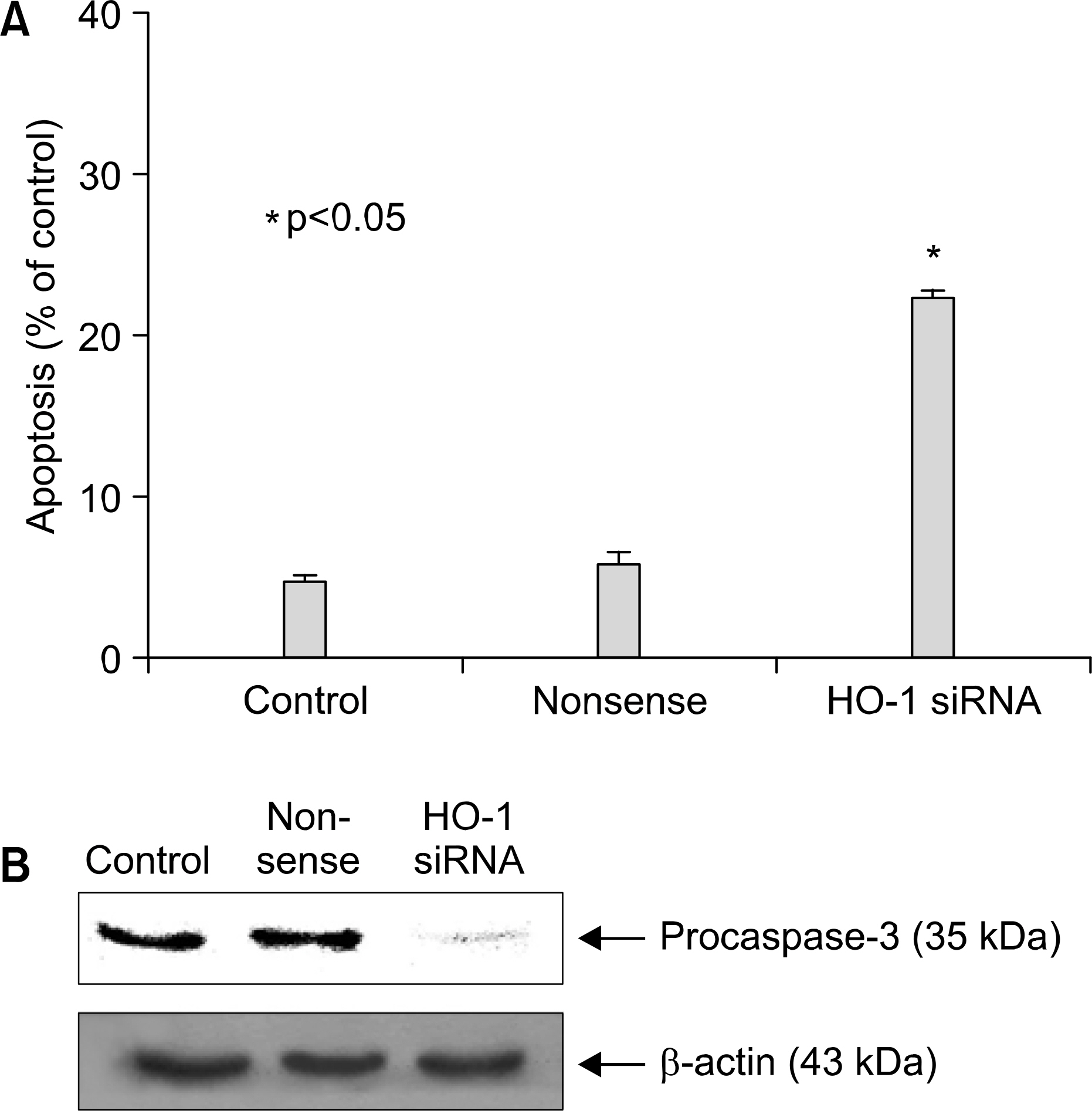
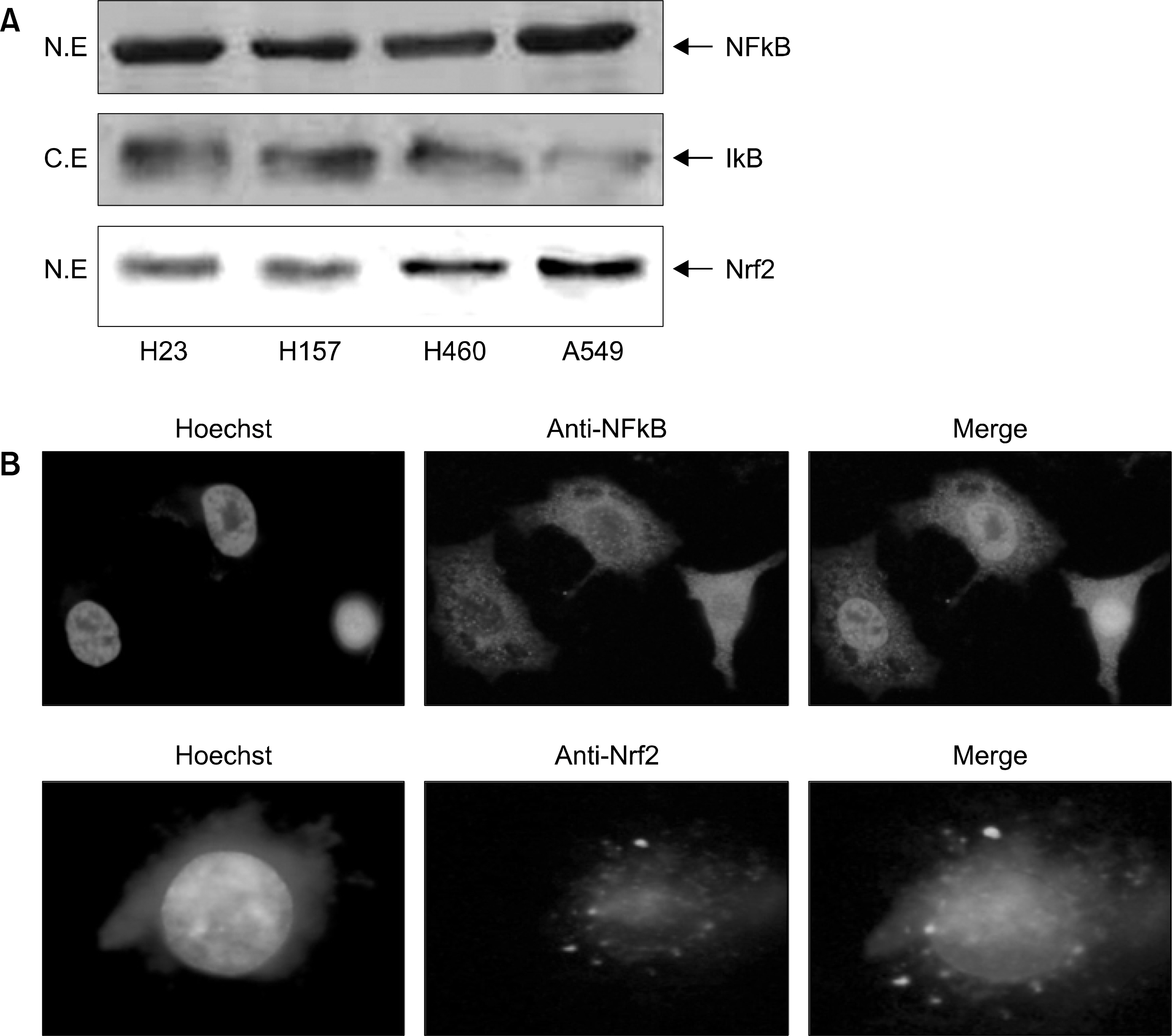
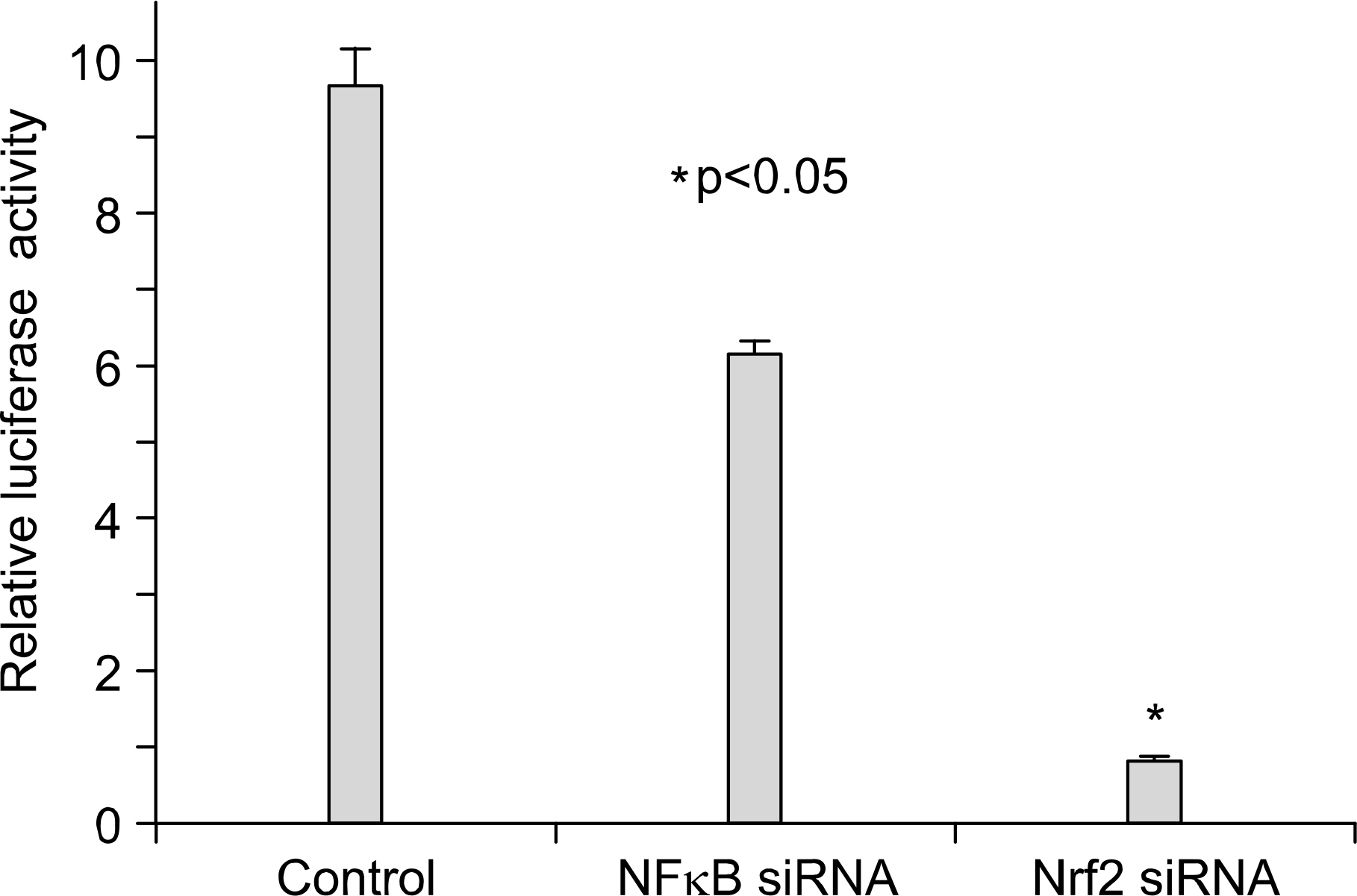
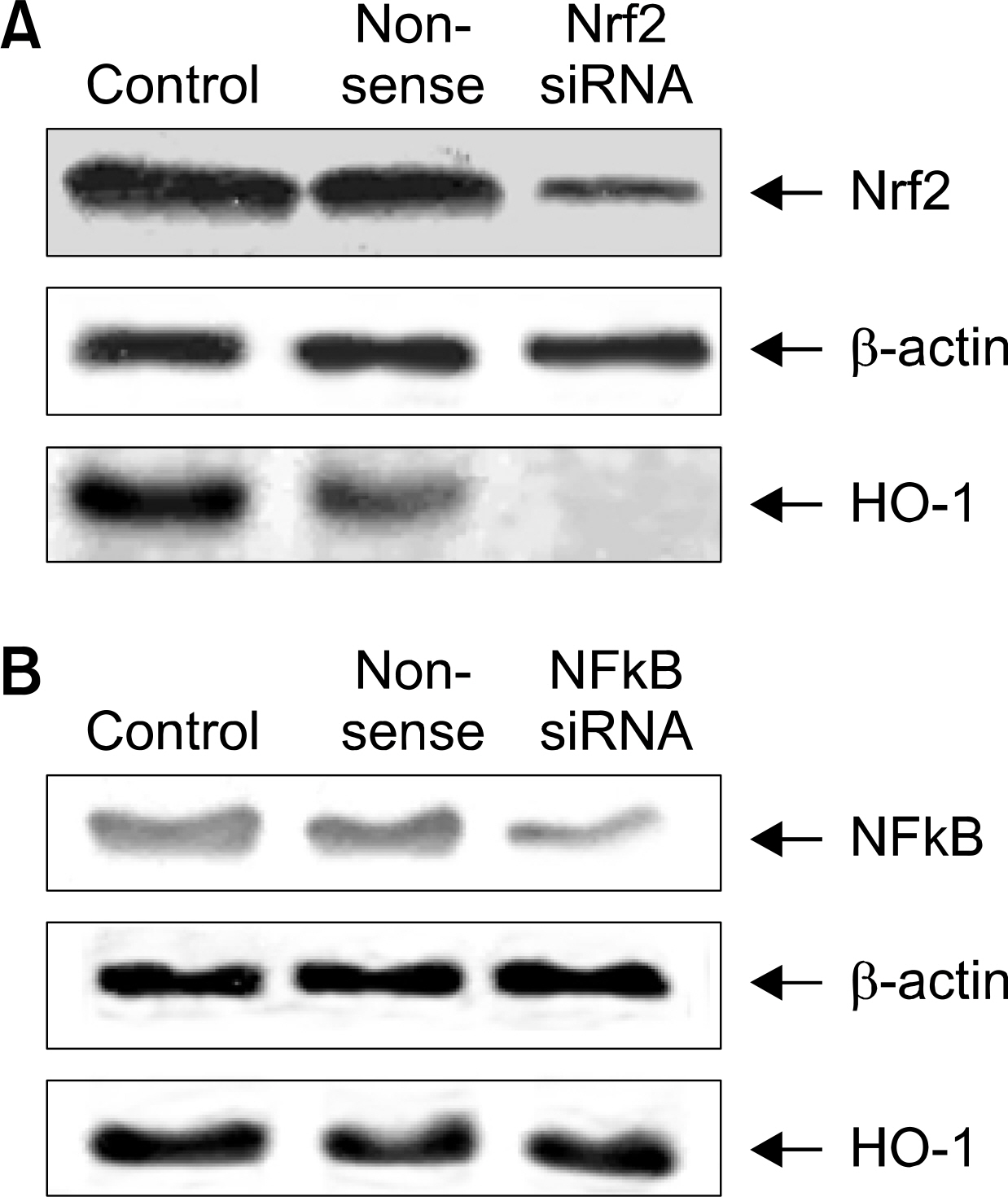
: We demonstrated that HO-1 was expressed highly in A549 cells than other lung cancer cells. And A549 cells were transfected by HO-1 small interfering RNA (siRNA) induced apoptosis. Nrf2 siRNA, next, resulted in a decrease of HO-1 expression. However, NFkB siRNA had no influence on the expression of HO-1.
CONCLUSION
: Increasing HO-1 expression in A549 cells may be resulted from the transcriptional activation of Nrf2, and inhibition of Nrf2-HO-1 pathway induces apoptosis. Therefore it provides new important insights into the possible molecular mechanism of the antitumor therapy
Keyword
MeSH Terms
Figure
Reference
-
1.Tenhunen R: Marver HS: Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970. 75:410–421.2.Baranano DE: Rao M: Ferris CD., Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002. 99:16093–16098.3.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997. 37:517–554.4.Tenhunen R., Marver HS., Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970. 75:410–421.5.Motterlini R., Foresti R., Bassi R., Calabrese V: Clark JE., Green CJ. Endothelial heme oxygenase-1 induction by hypoxia. Modulation by inducible nitric-oxide synthase and S-nitrosothiols. J Biol Chem. 2000. 275:13613–13620.6.Stuhlmeier KM. Activation and regulation of Hsp32 and Hsp 70. Eur J Biochem. 2000. 267:1161–1167.7.Doi K., Akaike T., Fujii S, et al. Induction of haem oxygenase-1 nitric oxide and ischaemia in experimental solid tumours and implications for tumour growth. Br J Cancer. 1999. 80:1945–1954.8.Elbirt KK: Whitmarsh AJ., Davis RJ: Bonkovsky HL. Mechanism of sodium arsenite-mediated induction of heme oxygenase-1 in hepatoma cells. Role of mitogen-activated protein kinases. J Biol Chem. 1998. 273:8922–8931.9.Eyssen-Hemandez R: Ladoux A., Frelin C. Differential regulation of cardiac heme oxygenase-1 and vascular endothelial growth factor mRNA expressions by hemin, heavy metals, heat shock and anoxia. FEBS Lett. 1996. 382:229–233.10.Keyse SM., Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci USA. 1989. 86:99–103.
Article11.Lautier D: Luscher P: Tyrrell RM. Endogenous glutathione levels modulate both constitutive and UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis. 1992. 13:227–232.
Article12.Hara E., Takahashi K., Takeda K, et al. Induction of heme oxygenase-1 as a response in sensing the signals evoked by distinct nitric oxide donors. Biochem Pharmacol. 1999. 58:227–236.
Article13.Rizzardini M., Terao M., Falciani F., Cantoni L. Cytokine induction of haem oxygenase mRNA in mouse liver. Interleukin 1 transcriptionally activates the haem oxygenase gene. Biochem J. 1993. 290:343–347.
Article14.Agarwal A., Shiraishi F., Visner GA., Nick HS. Linoleyl hydroperoxide transcriptionally upregulates heme oxygenase-1 gene expression in human renal epithelial and aortic endothelial cells. J Am Soc Nephrol. 1998. 9:1990–1997.
Article15.Fogg S., Agarwal A., Nick HS., Visner GA. Iron regulates hyperoxia-dependent human heme oxygenase 1 gene expression in pulmonary endothelial cells. Am J Respir Cell Mol Biol. 1999. 20:797–804. ,.
Article16.Alam J: Stewart D., Touchard C: Boinapally S: Choi AM: Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Bio Chem. 1999. 274:26071–26078.17.Wagner M., Cadetg P., Ruf R., Mazzucchelli L., Ferrari P., Redaelli CA. Heme oxygenase-1 attenuates ischemia/reperfusion-induced apoptosis and improves survival in rat renal allografts. Kidney Int. 2003. 63:1564–1573.
Article18.Choi BM., Pae HO., Chung HT. Nitric oxide priming protects nitric oxide-mediated apoptosis via heme oxygenase-1 induction. Free Radic Biol Med. 2003. 34:1136–1145.
Article19.Amon M., Menger MD., Vollmar B. Heme oxygenase and nitric oxide synthase mediate cooling-associated protection against TNF-alpha-induced microcirculatory dysfunction and apoptotic cell death. F ASEB J. 2003. 17:175–185.20.Ke B., Shen XD., Zhai Y, et al. Heme oxygenase 1 mediates the immunomodulatory and antiapoptotic effects of interleukin 13 gene therapy in vivo and in vitro. Hum Gene Ther. 2002. 13:1845–1857.21.Goodman Al., Choudhury M., da Silva JL., Schwartzman ML., Abraham NG. Overexpression of the heme oxygenase gene in renal cell carcinoma, Proc Soc Exp Biol Med. 1997. 214:54–61.22.Maines MD., Abrahamsson PA. Expression of heme oxygenase-1 (HSP32) in human prostate: normal, hyperplastic, and tumor tissue distribution. Urology. 1996. 47:727–733.
Article23.Sahoo SK., Sawa T., Fang J, et al. Pegylated zinc protopo卜phyrin: a water-soluble heme oxygenase inhibitor with tumortargeting capacity. Bioconjug Chem. 2002. 13:1031–1038.24.Crissman HA., Steinkamp JA. Cell cycle-related changes in chromatin structure detected by flow cytometry using multiple DNA fluorochromes. Eur J Histochem. 1993. 37:129–138.25.Elbashir SM: Harborth J: Lendeckel W., Yalcin A: Weber K., Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001. 411:494–498.
Article26.Alam J: Wicks C: Stewart D, et al. Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem. 2000. 275:27694–27702.27.He CH., Gong P., Hu B, et al. Identification of activating transcription factor 4 (ATF4) as an Nif2-interacting protein. Implication for heme oxygenase-1 gene regulation. J Biol Chem. 2001. 276:20858–20865.28.Banning A: Brigelius-Flohe R. NF-kappaB, Nrf2, and HO-1 interplay in redox reguhted VC AM 니expression. Antioxid Redox Signal. 2005. 7:889–899.29.Deramaudt BM., Braunstein S: Remy P: Abraham NG. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem. 1998. 68:121–127.
Article30.Hanselmann C: Mauch C., Werner S. Heme oxygenase-1: a novel player in cutaneous wound repair and psoriasis? Biochem J. 2001. 353:459–466.31.Fang J: Akaike T: Maeda H. Antiapoptotic role of heme oxygenase (HO) and the potential of HO as a target in anticancer treatment. Apoptosis. 2004. 9:27–35.
Article32.Fang J., Sawa T: Akaike T, et al. In vivo antitumor activity of pegylated zinc protoporphyrin: targeted inhibition of heme oxygenase in solid tumor. Cancer Res. 2003. 63:3567–3574.33.Sunamura M., Duda DG., Ghattas MH, et al. Heme oxygenase-1 accelerates tumor angiogenesis of human pancreatic cancer. Angiogenesis. 2003. 6:15–24.34.Tanaka S: Akaike T: Fang J, et al. Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumor. Br J Cancer. 2003. 88:902–909.35.Alam J: Stewart D., Touchard C., Boinapally S: Choi AM., Cook JL. Nrf2, a Cap'n'Cdlar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999. 274:26071–26078.36.Zipper LM: Mulcahy RT. The Keapl BTB/POZ dimerization function is required to sequester Nrf2 in cytoplasm. J Biol Chem. 2002. 277:36544–36552.37.Barkett M., Gilmore TD. Control of apoptosis by Rel/NF-kappa B transcription factors. Oncogene. 1999. 18:6910–6924.38.Liu ZM., Chen GG., Ng EK., Leung WK., Sung JJ., Chung SC. Upregulation of heme oxygenase-1 and p21 confers resistance to apoptosis in human gastric cancer cells. Oncogene. 2004. 23:503–513.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Inhibition of Heme Oxygenase-1 on Chemosensitivity of Cisplatin in Lung Cancer Cells
- Fraxetin Induces Heme Oxygenase-1 Expression by Activation of Akt/Nrf2 or AMP-activated Protein Kinase α/Nrf2 Pathway in HaCaT Cells
- Nrf2-Heme oxygenase-1 modulates autophagy and inhibits apoptosis triggered by elevated glucose levels in renal tubule cells
- Oxymatrine inhibits the pyroptosis in rat insulinoma cells by affecting nuclear factor kappa B and nuclear factor (erythroidderived 2)-like 2 protein/heme oxygenase-1 pathways
- Isoegomaketone Upregulates Heme Oxygenase-1 in RAW264.7 Cells via ROS/p38 MAPK/Nrf2 Pathway