Korean J Urol.
2012 Oct;53(10):726-732. 10.4111/kju.2012.53.10.726.
Synergistic Effect of Mesenchymal Stem Cells Infected with Recombinant Adenovirus Expressing Human BDNF on Erectile Function in a Rat Model of Cavernous Nerve Injury
- Affiliations
-
- 1Department of Urology, The Catholic University of Korea School of Medicine, Seoul, Korea. ksw1227@catholic.ac.kr
- 2Division of Molecular and Life Science, Integrative Bioscience and Biotechnology, WCU, Pohang University of Science and Technology (POSTECH), Pohang, Korea.
- KMID: 1856994
- DOI: http://doi.org/10.4111/kju.2012.53.10.726
Abstract
- PURPOSE
To evaluate the combined role of mescenchymal stem cells (MSCs) infected with recombinant adenoviruses expressing human BDNF (rAd/hBDNF) on the erectile dysfunction in rat with cavernous nerve injury.
MATERIALS AND METHODS
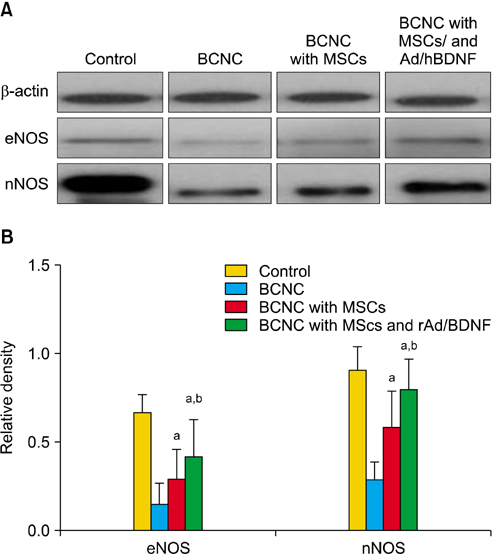
Rats divided into 4 groups: control group, bilateral cavernous nerve crushing group (BCNC group), BCNC with MSCs group and BCNC with MSCs infected with rAd/hBDNF group. After 4-week, functional assessment was done. PKH26 and BDNF staining of major pelvic ganglion and masson's trichrome staining of corpus cavernosum were performed. Western blot analysis of endothelial nitric oxide synthase (eNOS) and neuronal nitric oxide synthase (nNOS) was done in corpus cavernosum.
RESULTS
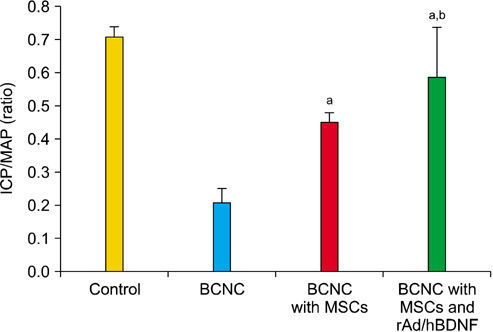
After 4 weeks, BCNC with MSCs and MSCs infected with rAd/hBDNF groups showed significantly well-preserved erectile function compared with BCNC group. Moreover, the erectile function of MSCs infected with rAd/hBDNF group was significantly well-preserved than BCNC with MSCs group. The smooth muscle of corpus cavernosum was significantly preserved in BCNC with MSCs and MSCs infected with rAd/hBDNF groups compared with BCNC group. More preservation of smooth muscle was observed in rats with MSCs infected with rAd/hBDNF than with MSCs alone. Significant increase expression of eNOS and nNOS was noted in rats with MSCs infected with rAd/hBDNF than with MSCs alone.
CONCLUSIONS
The erectile function was more preserved after injection with MSCs infected with rAd/hBDNF in rat with ED caused by cavernous nerve injury. Therefore, the use of MSC infected with rAd/hBDNF may have a better treatment effect on ED cause by cavernous nerve injury.
MeSH Terms
-
Adenoviridae
Animals
Blotting, Western
Brain-Derived Neurotrophic Factor
Caves
Erectile Dysfunction
Ganglion Cysts
Humans
Male
Mesenchymal Stromal Cells
Muscle, Smooth
Nerve Crush
Nitric Oxide Synthase Type I
Nitric Oxide Synthase Type III
Organic Chemicals
Rats
Stem Cells
Brain-Derived Neurotrophic Factor
Nitric Oxide Synthase Type I
Nitric Oxide Synthase Type III
Organic Chemicals
Figure
Cited by 1 articles
-
The Current Status of Stem-Cell Therapy in Erectile Dysfunction: A Review
Amanda B Reed-Maldonado, Tom F Lue
World J Mens Health. 2016;34(3):155-164. doi: 10.5534/wjmh.2016.34.3.155.
Reference
-
1. Shao YH, Demissie K, Shih W, Mehta AR, Stein MN, Roberts CB, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009. 101:1280–1283.2. Anastasiadis AG, Salomon L, Katz R, Hoznek A, Chopin D, Abbou CC. Radical retropubic versus laparoscopic prostatectomy: a prospective comparison of functional outcome. Urology. 2003. 62:292–297.3. Roumeguere T, Bollens R, Vanden Bossche M, Rochet D, Bialek D, Hoffman P, et al. Radical prostatectomy: a prospective comparison of oncological and functional results between open and laparoscopic approaches. World J Urol. 2003. 20:360–366.4. Krambeck AE, DiMarco DS, Rangel LJ, Bergstralh EJ, Myers RP, Blute ML, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009. 103:448–453.5. Stephenson RA, Mori M, Hsieh YC, Beer TM, Stanford JL, Gilliland FD, et al. Treatment of erectile dysfunction following therapy for clinically localized prostate cancer: patient reported use and outcomes from the Surveillance, Epidemiology, and End Results Prostate Cancer Outcomes Study. J Urol. 2005. 174:646–650.6. Walsh PC, Partin AW, Epstein JI. Cancer control and quality of life following anatomical radical retropubic prostatectomy: results at 10 years. J Urol. 1994. 152(5 Pt 2):1831–1836.7. Haffner MC, Landis PK, Saigal CS, Carter HB, Freedland SJ. Health-related quality-of-life outcomes after anatomic retropubic radical prostatectomy in the phosphodiesterase type 5 ERA: impact of neurovascular bundle preservation. Urology. 2005. 66:371–376.8. Raina R, Pahlajani G, Agarwal A, Zippe CD. Early penile rehabilitation following radical prostatectomy: Cleveland clinic experience. Int J Impot Res. 2008. 20:121–126.9. Zippe CD, Pahlajani G. Penile rehabilitation following radical prostatectomy: role of early intervention and chronic therapy. Urol Clin North Am. 2007. 34:601–618. viii10. Harraz A, Shindel AW, Lue TF. Emerging gene and stem cell therapies for the treatment of erectile dysfunction. Nat Rev Urol. 2010. 7:143–152.11. Bakircioglu ME, Lin CS, Fan P, Sievert KD, Kan YW, Lue TF. The effect of adeno-associated virus mediated brain derived neurotrophic factor in an animal model of neurogenic impotence. J Urol. 2001. 165(6 Pt 1):2103–2109.12. Park SH, Doh J, Park SI, Lim JY, Kim SM, Youn JI, et al. Branched oligomerization of cell-permeable peptides markedly enhances the transduction efficiency of adenovirus into mesenchymal stem cells. Gene Ther. 2010. 17:1052–1061.13. Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol. 2000. 28:875–884.14. Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008. 180:2581–2587.15. Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci U S A. 2000. 97:12846–12851.16. Levy YS, Bahat-Stroomza M, Barzilay R, Burshtein A, Bulvik S, Barhum Y, et al. Regenerative effect of neural-induced human mesenchymal stromal cells in rat models of Parkinson's disease. Cytotherapy. 2008. 10:340–352.17. Bella AJ, Lin G, Lin CS, Hickling DR, Morash C, Lue TF. Nerve growth factor modulation of the cavernous nerve response to injury. J Sex Med. 2009. 6:Suppl 3. 347–352.18. Bella AJ, Lin G, Tantiwongse K, Garcia M, Lin CS, Brant W, et al. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part I. J Sex Med. 2006. 3:815–820.19. Lin G, Bella AJ, Lue TF, Lin CS. Brain-derived neurotrophic factor (BDNF) acts primarily via the JAK/STAT pathway to promote neurite growth in the major pelvic ganglion of the rat: part 2. J Sex Med. 2006. 3:821–827.20. Bella AJ, Lin G, Garcia MM, Tantiwongse K, Brant WO, Lin CS, et al. Upregulation of penile brain-derived neurotrophic factor (BDNF) and activation of the JAK/STAT signalling pathway in the major pelvic ganglion of the rat after cavernous nerve transection. Eur Urol. 2007. 52:574–580.21. Yaghoobi MM, Mowla SJ. Differential gene expression pattern of neurotrophins and their receptors during neuronal differentiation of rat bone marrow stromal cells. Neurosci Lett. 2006. 397:149–154.22. Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, et al. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007. 27:355–363.23. Yaghoobi MM, Mahani MT. NGF and BDNF expression drop off in neurally differentiated bone marrow stromal stem cells. Brain Res. 2008. 1203:26–31.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transplantation of Muscle-Derived Stem Cells into the Corpus Cavernosum Restores Erectile Function in a Rat Model of Cavernous Nerve Injury
- Use of nanoparticles to monitor human mesenchymal stem cells transplanted into penile cavernosum of rats with erectile dysfunction
- Efficacy of a Red-Light Controllable Nitric Oxide Releaser for Neurogenic Erectile Dysfunction: A Study Using a Rat Model of Cavernous Nerve Injury
- Adipose Derived Mesenchymal Stem Cells-Derived Mitochondria Transplantation Ameliorated Erectile Dysfunction Induced by Cavernous Nerve Injury
- Stem Cell Therapy for Erectile Dysfunction