J Korean Orthop Assoc.
2015 Jun;50(3):241-248. 10.4055/jkoa.2015.50.3.241.
Influence of Recombinant Human Bone Morphogenetic Protein-2 on the Remodeling of Subchondral Bone and Cartilage Healing in the Articular Cartilage Defect of the Rabbit
- Affiliations
-
- 1Department of Orthopedic Surgery, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea. lsjmd@kuh.ac.kr
- 2Department of Orthopedic Surgery, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea.
- KMID: 1851958
- DOI: http://doi.org/10.4055/jkoa.2015.50.3.241
Abstract
- PURPOSE
The purpose of this study is to evaluate the effects of recombinant human bone morphogenetic protein-2 (rhBMP-2) after microfracture on the remodeling of subchondral bone and cartilage healing in a model of full-thickness articular cartilage injury in a rabbit.
MATERIALS AND METHODS
A full thickness articular cartilage defect of 6x3-mm-size was created in the trochlear groove of the right femur in 24 rabbits. The defect was left empty in six rabbits, and microfracture was done in 18 rabbits. After microfracture, no treatment was done in six rabbits, defect was filled with fibrin glue in six rabbits, and with fibrin glue and rhBMP-2 in six rabbits. The effect of rhBMP-2 after microfracture was evaluated based on histological analysis and real-time polymerase chain reaction (PCR) for analysis of collagen type at 8 weeks after surgery.
RESULTS
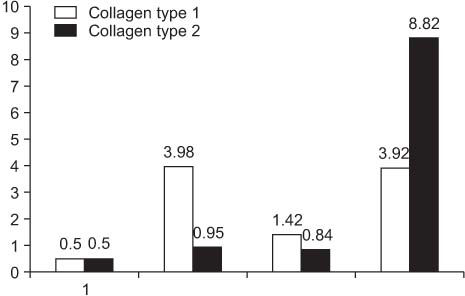
The score of histological grade scale of six rabbits in which the defect was filled with fibrin glue and rhBMP-2 was better than that of others and real-time PCR also showed a higher amount of collage type 1 and collage type 2 in these six rabbits.
CONCLUSION
We consider that fibrin glue and rhBMP-2 after microfracture may accelerate cartilage healing in an articular cartilage defect and maybe helpful in healing the articular cartilage defect into more closely native hyaline cartilage.
MeSH Terms
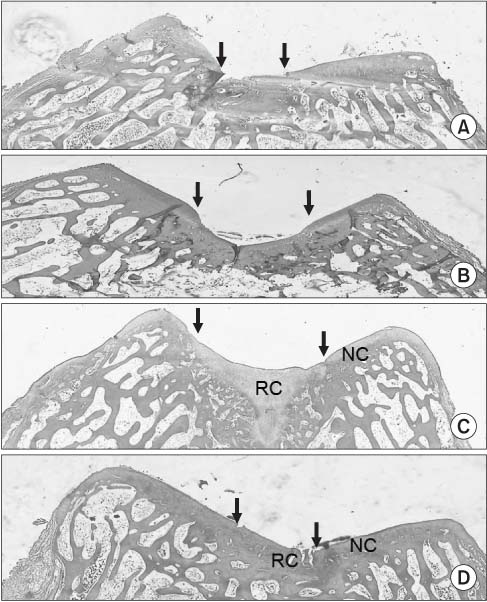
Figure
Reference
-
1. Urist MR. Bone: formation by autoinduction. Science. 1965; 150:893–899.2. Bostrom M, Lane JM, Tomin E, et al. Use of bone morphogenetic protein-2 in the rabbit ulnar nonunion model. Clin Orthop Relat Res. 1996; 327:272–282.3. Jones AL, Bucholz RW, Bosse MJ, et al. BMP-2 Evaluation in Surgery for Tibial Trauma-Allgraft (BESTT-ALL) Study Group. Recombinant human BMP-2 and allograft compared with autogenous bone graft for reconstruction of diaphyseal tibial fractures with cortical defects. A randomized, controlled trial. J Bone Joint Surg Am. 2006; 88:1431–1441.4. Boyne PJ, Lilly LC, Marx RE, et al. De novo bone induction by recombinant human bone morphogenetic protein-2 (rh-BMP-2) in maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2005; 63:1693–1707.5. McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop. 2007; 31:729–734.6. Siebert CH, Schneider U, Sopka S, Wahner T, Miltner O, Niedhart C. Ingrowth of osteochondral grafts under the influence of growth factors: 6-month results of an animal study. Arch Orthop Trauma Surg. 2006; 126:247–252.7. Siebert CH, Miltner O, Weber M, Sopka S, Koch S, Niedhart C. Healing of osteochondral grafts in an ovine model under the influence of bFGF. Arthroscopy. 2003; 19:182–187.8. Wim B, Peter M, Scharstuhl A, Henk M. Tissue engineering, cells, scaffolds, and growth factors. Clin Orthop Relat Res. 2001; 391:244–250.9. Pineda S, Pollack A, Stevenson S, Goldberg V, Caplan A. A semiquantitative scale for histologic grading of articular cartilage repair. Acta Anat (Basel). 1992; 143:335–340.10. Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982; 64:460–466.11. Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998; 28:192–202.12. Wei X, Gao J, Messner K. Maturation-dependent repair of untreated osteochondral defects in the rabbit knee joint. J Biomed Mater Res. 1997; 34:63–72.13. Steadman JR, Rodkey WG, Singleton SB, Britts KK. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop. 1997; 7:300–304.14. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003; 19:477–484.15. Blevins FT, Steadman JR, Rodrigo JJ, Silliman J. Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. Orthopedics. 1998; 21:761–767.16. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001; 391 Suppl. S362–S369.17. Frisbie DD, Trotter GW, Powers BE, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999; 28:242–255.18. Steadman RJ, Rodkey WG, Singleton SB, et al. Microfracture procedure for treatment of full-thickness chondral defects: technique, clinical results, and current basic science status. In : Harner CD, Vince KG, Fu FH, editors. Techniques in knee surgery. Philadelphia: Lippincott Williams & Wilkins;2001. p. 23–31.19. Kang SW, Bada LP, Kang CS, et al. Articular cartilage regeneration with microfracture and hyaluronic acid. Biotechnol Lett. 2008; 30:435–439.20. Ishii I, Mizuta H, Sei A, Hirose J, Kudo S, Hiraki Y. Healing of full-thickness defects of the articular cartilage in rabbits using fibroblast growth factor-2 and a fibrin sealant. J Bone Joint Surg Br. 2007; 89:693–700.21. Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cartilage. 2006; 14:1126–1135.22. Katagiri T, Yamaguchi A, Ikeda T, et al. The non-osteogenic mouse pluripotent cell line, C3H10T1/2, is induced to differentiate into osteoblastic cells by recombinant human bone morphogenetic protein-2. Biochem Biophys Res Commun. 1990; 172:295–299.23. Thies RS, Bauduy M, Ashton BA, Kurtzberg L, Wozney JM, Rosen V. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992; 130:1318–1324.24. Li B, Aspden RM. Mechanical and material properties of the subchondral bone plate from the femoral head of patients with osteoarthritis or osteoporosis. Ann Rheum Dis. 1997; 56:247–254.25. Conaghan PG, Vanharanta H, Dieppe PA. Is progressive osteoarthritis an atheromatous vascular disease? Ann Rheum Dis. 2005; 64:1539–1541.26. Shibakawa A, Yudoh K, Masuko-Hongo K, Kato T, Nishioka K, Nakamura H. The role of subchondral bone resorption pits in osteoarthritis: MMP production by cells derived from bone marrow. Osteoarthritis Cartilage. 2005; 13:679–687.27. Thomopoulos S, Soslowsky LJ, Flanagan CL, et al. The effect of fibrin clot on healing rat supraspinatus tendon defects. J Shoulder Elbow Surg. 2002; 11:239–247.28. Lusardi DA, Cain JE Jr. The effect of fibrin sealant on the strength of tendon repair of full thickness tendon lacerations in the rabbit Achilles tendon. J Foot Ankle Surg. 1994; 33:443–447.29. Stemberger A, Blümel G. Fibrinogen-fibrin conversion and inhibition of fibrinolysis. Thorac Cardiovasc Surg. 1982; 30:209–214.30. Schlag G, Redl H. Fibrin sealant in orthopedic surgery. Clin Orthop Relat Res. 1988; 227:269–285.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autogeous Bone-Articular Cartilage stored within Abdominal Wall
- Regeneration of Full - Thickness Defect of Articular Cartilage in Rabbit
- The Effect of the Small amount of Intra-articular Hydrocortisone on the Repair of Injured Articular Cartilge
- An experimental intraarticular implantation of woven carbon fiber pad into osteochondral defect of the femoral condyle in rabbit
- Regulation of Articular Cartilage Defects with OP-1 Treated Lyphilized Cartilage Allografts