J Clin Neurol.
2007 Jun;3(2):82-85. 10.3988/jcn.2007.3.2.82.
One-Year Open-Label Study of Entacapone in Patients with Advanced Parkinson Disease
- Affiliations
-
- 1Department of Neurology, Kyung Hee University College of Medicine, Korea.
- 2Department of Neurology, Ulsan University College of Medicine, Korea.
- 3Department of Neurology, Dong-A University School of Medicine, Korea.
- 4Department of Neurology, Sungkyunkwan University School of Medicine, Korea.
- 5Department of Neurology, Seoul National University College of Medicine, Korea. brain@snu.ac.kr
- KMID: 1851017
- DOI: http://doi.org/10.3988/jcn.2007.3.2.82
Abstract
- BACKGROUND AND PURPOSE
A carboxy-O-methyl transferase inhibitor entacapone has been introduced as an adjuvant drug for Parkinson disease (PD) patients. Although clinical trials reported beneficial role of entacapone, a long-term trial over 3 years failed to show significant effect. The goals of this study were to evaluate the clinical benefit and the efficacy of entacapone in an open clinical practice.
METHODS
After the completion of a double-blind placebo-controlled entacapone study, 149 patients from 4 centers were included. Antiparkinsonian medications were optimized by the judgment of the neurologists in charge. The clinical global impression (CGI) scale was obtained at 6 months and 1 year after the initiation of entacapone treatment.
RESULTS
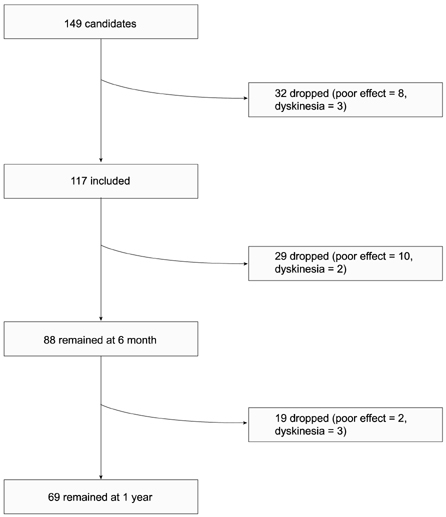
Of the 149 patients, 117 patients chose to try entacapone in an open-label fashion. Sixty-nine (59%) patients completed the 1-year trial. Twenty-nine patients discontinued entacpaone before 6 months, and 19 between 6 months and 1 year during trial. Twelve patients out of 48 patients discontinued entacapone because of its poor efficacy. The CGI scale was 3.9 (+/-1.5) at the beginning of the trial, 4.3 (+/-1.1) at 6 month, and 3.8 (+/-1.3) at 1 year, respectively. The CGI scale of those who discontinued between 6 month and 1 year was 3.4 (+/-1.7), which was worse, but insignificantly, than that of the continuer.
CONCLUSIONS
The dropout at 1 year of our study was very high at 41%. Even though entacapone is indicated for advanced PD patients with motor fluctuation, the fluctuators commonly have dyskinesia and mental symptoms, which can become more troublesome with entacapone. In the patients with advanced PD, the clinical efficacy and side effects should be carefully considered in a long-term use of entacapone.
Keyword
Figure
Reference
-
1. Parkinson study group. Entacapone improves motor fluctuations in levodopa-treated Parkinson's disease patients. Ann Neurol. 1997. 42:747–755.2. Rinne UK, Larsen JP, Siden A, Worm-Petersen J; Nomecomtstudy group. Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Neurology. 1998. 51:1309–1314.
Article3. Ruottinen HM, Rinne UK. Entacapone prolongs levodopa response in a one-month double-blind study in parkinsonian patients with levodopa related fluctuations. J Neurol Neurosurg Psychiatry. 1996. 60:36–40.
Article4. Larsen JP, Worm-Peterson J, Siden A, Gordin A, Reinkainen K. The tolerability and efficacy of entacapone over 3 years in patients with Parkinson's disease. Eur J Neurol. 2003. 10:137–146.
Article5. Fenelon G, Gimenez-Roldan S, Mostastrue JL, Bermejo F, Durif F, Bourdeix I, et al. Efficacy and tolerability of entacapone in patients with Parkinson's disease treated with levodopa plus a dopamine agonist and experiencing wearing-off motor fluctuations. A randomized, doubleblind, multicentre study. J Neural Trans. 2003. 110:239–251.
Article6. Im JH, Lee JK, Chung SJ, Jeon BS, Cho JH, Lee MS, et al. Efficacy and Safety of Entacapone in the Patients with Parkinson's Disease Experiencing Wearing-off Phenomenon: Multicenter Randomized Placebo-controlled Double Blind Study. J Korean Neurol Assoc. 2005. 23:206–214.7. Guy W. ECDEU Assessment for Psychopharmacology. 1976. Revised Edition. Rockville: NIMH Publication;218–222.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In silico discovery and evaluation of phytochemicals binding mechanism against human catechol-Omethyltransferase as a putative bioenhancer of L-DOPA therapy in Parkinson disease
- Efficacy and Safety of Entacapone in the Patients with Parkinson's Disease Experiencing Wearing-off Phenomenon: Multicenter Randomized Placebo-controlled Double Blind Study
- Therapeutic Effect of Levodopa/Carbidopa/Entacapone on Sleep Disturbance in Patients with Parkinson’s Disease
- The Effects of Zolpidem on Akinesia in a Parkinson Disease Patient: A Case Report
- Purposeless Groaning in Parkinson's Disease