Lab Anim Res.
2011 Sep;27(3):255-258. 10.5625/lar.2011.27.3.255.
Sensitive and specific identification by polymerase chain reaction of Eimeria tenella and Eimeria maxima, important protozoan pathogens in laboratory avian facilities
- Affiliations
-
- 1Center for Animal Resources Development, Wonkwang University, Iksan, Korea. kimoj@wku.ac.kr
- 2Institute of Animal Experiment & Efficacy Evaluation, Wonkwang University, Iksan, Korea.
- 3Department of Companion Animal and Animal Recourses Science, Joongbu University, Geumsan, Korea.
- 4Institute of Biotechnology, Wonkwang University, Iksan, Korea.
- KMID: 1850054
- DOI: http://doi.org/10.5625/lar.2011.27.3.255
Abstract
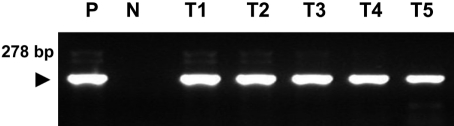
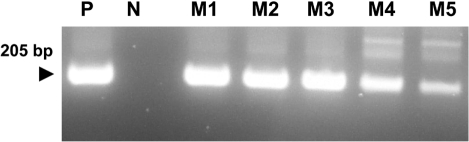
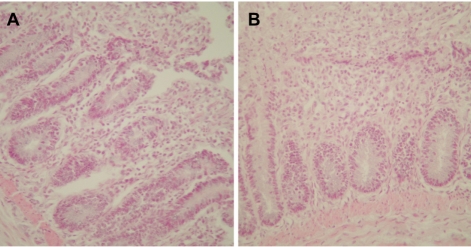
- Eimeria tenella and Eimeria maxima are important pathogens causing intracellular protozoa infections in laboratory avian animals and are known to affect experimental results obtained from contaminated animals. This study aimed to find a fast, sensitive, and efficient protocol for the molecular identification of E. tenella and E. maxima in experimental samples using chickens as laboratory avian animals. DNA was extracted from fecal samples collected from chickens and polymerase chain reaction (PCR) analysis was employed to detect E. tenella and E. maxima from the extracted DNA. The target nucleic acid fragments were specifically amplified by PCR. Feces secreting E. tenella and E. maxima were detected by a positive PCR reaction. In this study, we were able to successfully detect E. tenella and E. maxima using the molecular diagnostic method of PCR. As such, we recommended PCR for monitoring E. tenella and E. maxima in laboratory avian facilities.
MeSH Terms
Figure
Reference
-
1. Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev Vaccines. 2006; 5(1):143–163. PMID: 16451116.
Article2. Wilson PA, Fairbairnz D. Biochemistry of sporulation in oocysts of Eimeria acervulina. J Protozool. 1961; 8(4):410–416.3. Long PL, Millard BJ, Joyner LP, Norton CC. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat. 1976; 6(3):201–217. PMID: 1010500.4. Long PL, Reid WM. A guide for the diagnosis of coccidiosis in chickens. Research report. 1982. 404:The University of Georgia, College of Agriculture Experiment Stations;p. 1–17.5. Long PL, Joyner LP. Problems in identification of species of Eimeria. J Protozool. 1984; 31(4):535–541. PMID: 6392531.6. Stucki U, Braun R, Roditi I. Eimeria tenella: characterization of a 5S ribosomal RNA repeat unit and its use as a species-specific probe. Exp Parasitol. 1993; 76(1):68–75. PMID: 8467900.
Article7. Tsuji N, Ohta M, Kawazu S, Kamio T, Isobe T, Shimura K, Fujisaki K. DNA polymorphism of srRNA gene among Eimeria tenella strains isolated in Japan. J Vet Med Sci. 1999; 61(12):1331–1333. PMID: 10651056.
Article8. Molloy JB, Eaves FW, Jeston PJ, Minchin CM, Stewart NP, Lew AE, Jorgensen WK. Detection of Eimeria acervulina using the polymerase chain reaction. Avian Dis. 1998; 42(1):119–123. PMID: 9533088.9. Lew AE, Anderson GR, Minchin CM, Jeston PJ, Jorgensen WK. Inter- and intra-strain variation and PCR detection of the internal transcribed spacer 1 (ITS-1) sequences of Australian isolates of Eimeria species from chickens. Vet Parasitol. 2003; 112(1-2):33–50. PMID: 12581583.
Article10. Schnitzler BE, Thebo PL, Mattsson JG, Tomley FM, Shirley MW. Development of a diagnostic PCR assay for the detection and discrimination of four pathogenic Eimeria species of the chicken. Avian Pathol. 1998; 27(5):490–497. PMID: 18484033.11. Schnitzler BE, Thebo PL, Tomley FM, Uggla A, Shirley MW. PCR identification of chicken Eimeria: a simplified read-out. Avian Pathol. 1999; 28(1):89–93. PMID: 16147553.
Article12. Su YC, Fei AC, Tsai FM. Differential diagnosis of five avian Eimeria species by polymerase chain reaction using primers derived from the internal transcribed spacer 1 (ITS-1) sequence. Vet Parasitol. 2003; 117(3):221–227. PMID: 14630430.
Article13. Gasser RB, Woods WG, Wood JM, Ashdown L, Richards G, Whithear KG. Automated, fluorescence-based approach for the specific diagnosis of chicken coccidiosis. Electrophoresis. 2001; 22(16):3546–3550. PMID: 11669540.
Article14. Lien YY, Sheu SC, Liu HJ, Chen SC, Tsai MY, Luo SC, Wu KC, Liu SS, Su HY. Cloning and nucleotide sequencing of the second internal transcribed spacer of ribosomal DNA for three species of Eimeria from chickens in Taiwan. Vet J. 2007; 173(1):184–189. PMID: 16314128.
Article15. Woods WG, Whithear KG, Richards DG, Anderson GR, Jorgensen WK, Gasser RB. Single-strand restriction fragment length polymorphism analysis of the second internal transcribed spacer (ribosomal DNA) for six species of Eimeria from chickens in Australia. Int J Parasitol. 2000; 30(9):1019–1023. PMID: 10980293.
Article16. Fernandez S, Pagotto AH, Furtado MM, Katsuyama AM, Madeira AM, Gruber A. A multiplex PCR assay for the simultaneous detection and discrimination of the seven Eimeria species that infect domestic fowl. Parasitology. 2003; 127(Pt 4):317–325. PMID: 14636018.17. Ryley JF. Hammond DM, editor. Cytochemistry, physiology, and biochemistry. The Coccidia. 1973. Baltimore: University Park Press;p. 151–154.18. Abrahamsen MS, Clark TG, White MW. An improved method for isolating RNA from coccidia n oocysts. J Parasitol. 1995; 81(1):107–109. PMID: 7876962.19. Jinneman KC, Wetherington JH, Hill WE, Adams AM, Johnson JM, Tenge BJ, Dang NL, Manger RL, Wekell MM. Template preparation for PCR and RFLP of amplification products for the detection and identification of Cyclospora sp. and Eimeria spp. oocysts directly from raspberries. J Food Prot. 1998; 61(11):1497–1503. PMID: 9829192.
Article20. Nakamura T, Konishi T, Kawaguchi H. Isoenzymes of chicken coccidia in Japan. Nihon Juigaku Zasshi. 1986; 48:587–590. PMID: 3735889.21. MacPherson JM, Gajadhar AA. Differentiation of seven Eimeria species by random amplified polymorphic DNA. Vet Parasitol. 1993; 45(3-4):257–266. PMID: 8447068.
Article22. Procunier JD, Fernando MA, Barta JR. Species and strain differentiation of Eimeria spp. of the domestic fowl using DNA polymorphisms amplified by arbitrary primers. Parasitol Res. 1993; 79(2):98–102. PMID: 8475039.23. Shirley MW. Enzyme variation in Eimeria species of the chicken. Parasitology. 1975; 71(3):369–376. PMID: 1202411.
Article24. McDougald LR. Saif YM, editor. Protozoal infections. Diseases of Poultry. 2003. Ames: Iowa State Press;p. 973–991.
Article25. Reid WM, Long PL. A diagnostic chart for nine species of fowl coccidian. Univ Ga Coll Agric Res Rep. 1979; 335:1–24.26. Stotish RL, Wang CC, Meyenhofer M. Structure and composition of the oocyst wall of Eimeria tenella. J Parasitol. 1978; 64(6):1074–1081. PMID: 739302.
Article27. Zhao X, Duszynski DW, Loker ES. A simple method of DNA extraction for Eimeria species. J Microbiol Methods. 2001; 44(2):131–137. PMID: 11165342.
Article28. Shirley MV. Eckert J, editor. Eimeria species and strains. COST89/820, Biotechnology, Guidelines on Techniques in Coccidiosis Research. 1995. Luxembourg: European Commission;p. 1–25.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative analysis of evaluation parameters in E. acervulina, E. maxima and E. tenella-infected broilers
- Anticoccidial Activity of Berberine against Eimeria-Infected Chickens
- Anticoccidial effects of Galla rhois extract on Eimeria tenella-infected chicken
- Suppression of Eimeria tenella Sporulation by Disinfectants
- Anticoccidial effects of the Plantago asiatica extract on experimental Eimeria tenella infection