Korean J Urol.
2013 Aug;54(8):547-554. 10.4111/kju.2013.54.8.547.
Possible Role of Sonic Hedgehog and Epithelial-Mesenchymal Transition in Renal Cell Cancer Progression
- Affiliations
-
- 1Department of Urology, Kobe University Graduate School of Medicine, Kobe, Japan. yutoshunta@hotmail.co.jp
- 2Faculty of Medicine, Assiut University, Assiut, Egypt.
- 3Infectious Center, Kobe University Graduate School of Medicine, Kobe, Japan.
- 4Department of Urology, Shinko Hospital, Kobe, Japan.
- 5Department of Medicine, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
- KMID: 1840469
- DOI: http://doi.org/10.4111/kju.2013.54.8.547
Abstract
- PURPOSE
Sonic hedgehog (Shh) signaling and epithelial-mesenchymal transition (EMT) are both known to relate to cancer progression. The purpose of this study was to investigate the role of Shh signaling and EMT in renal cell carcinoma (RCC).
MATERIALS AND METHODS
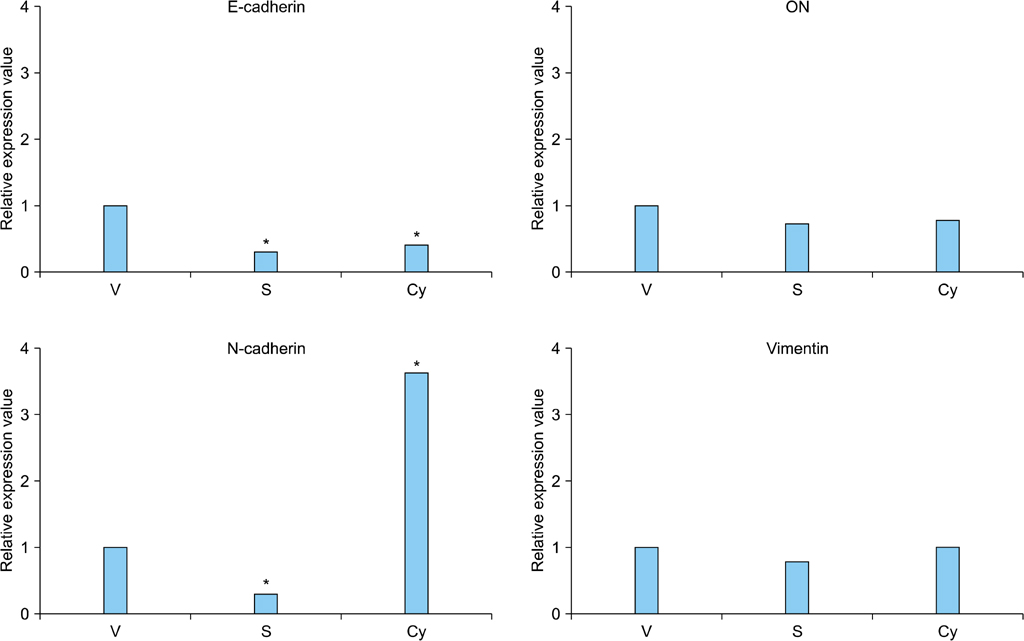
Cell proliferation was assayed in RCC cell lines in the presence or absence of a Shh signaling stimulator, recombinant Shh (r-Shh) protein, or a Shh signaling inhibitor, cyclopamine. Real-time reverse transcription-polymerase chain reaction (RT-PCR) was performed to study the expression of EMT markers (E-cadherin, N-cadherin, and vimentin) and osteonectin. The expression of Ki-67, Gli-1, osteonectin, and EMT markers in nephrectomy specimens from RCC patients was also measured by immunohistochemical (IHC) staining.
RESULTS
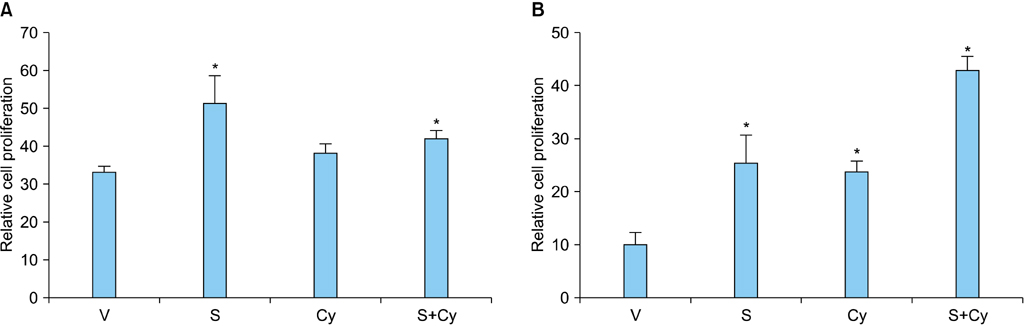
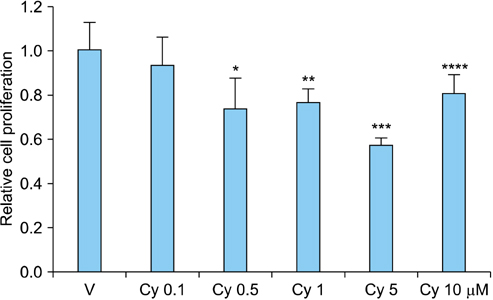
RCC cells showed enhanced cell proliferation by r-Shh protein, whereas cell proliferation was suppressed by the addition of cyclopamine in RenCa cells. Real-time RT-PCR showed that r-Shh suppressed the expression of E-cadherin and that this suppression was partly blocked by cyclopamine alone in RenCa cells. In the IHC results, osteonectin significantly correlated with vein sinus invasion (p=0.0218), and the expression of vimentin significantly correlated with lymphatic invasion (p=0.0392).
CONCLUSIONS
Shh signaling and EMT play roles in RCC progression, and the Shh signaling inhibitor cyclopamine might be a possible molecular targeted therapeutic strategy for RCC.
MeSH Terms
Figure
Reference
-
1. Cho IC, Chung J. Current status of targeted therapy for advanced renal cell carcinoma. Korean J Urol. 2012. 53:217–228.2. Sanchez P, Hernandez AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci U S A. 2004. 101:12561–12566.3. Datta S, Datta MW. Sonic Hedgehog signaling in advanced prostate cancer. Cell Mol Life Sci. 2006. 63:435–448.4. Dormoy V, Danilin S, Lindner V, Thomas L, Rothhut S, Coquard C, et al. The sonic hedgehog signaling pathway is reactivated in human renal cell carcinoma and plays orchestral role in tumor growth. Mol Cancer. 2009. 8:123.5. Li N, Felber K, Elks P, Croucher P, Roehl HH. Tracking gene expression during zebrafish osteoblast differentiation. Dev Dyn. 2009. 238:459–466.6. Guweidhi A, Kleeff J, Adwan H, Giese NA, Wente MN, Giese T, et al. Osteonectin influences growth and invasion of pancreatic cancer cells. Ann Surg. 2005. 242:224–234.7. Shigemura K, Huang WC, Li X, Zhau HE, Zhu G, Gotoh A, et al. Active sonic hedgehog signaling between androgen independent human prostate cancer cells and normal/benign but not cancer-associated prostate stromal cells. Prostate. 2011. 71:1711–1722.8. Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res. 2010. 3:90–99.9. Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012. 148:349–361.10. Lim M, Chuong CM, Roy-Burman P. PI3K, Erk signaling in BMP7-induced epithelial-mesenchymal transition (EMT) of PC-3 prostate cancer cells in 2- and 3-dimensional cultures. Horm Cancer. 2011. 2:298–309.11. Xu X, Zhou Y, Xie C, Wei SM, Gan H, He S, et al. Genome-wide screening reveals an EMT molecular network mediated by Sonic hedgehog-Gli1 signaling in pancreatic cancer cells. PLoS One. 2012. 7:e43119.12. Joost S, Almada LL, Rohnalter V, Holz PS, Vrabel AM, Fernandez-Barrena MG, et al. GLI1 inhibition promotes epithelial-to-mesenchymal transition in pancreatic cancer cells. Cancer Res. 2012. 72:88–99.13. Onishi T, Ogawa T, Hayashibara T, Hoshino T, Okawa R, Ooshima T. Hyper-expression of osteocalcin mRNA in odontoblasts of Hyp mice. J Dent Res. 2005. 84:84–88.14. Matsuoka TA, Kaneto H, Stein R, Miyatsuka T, Kawamori D, Henderson E, et al. MafA regulates expression of genes important to islet beta-cell function. Mol Endocrinol. 2007. 21:2764–2774.15. Aoi J, Endo M, Kadomatsu T, Miyata K, Nakano M, Horiguchi H, et al. Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 2011. 71:7502–7512.16. Kurahashi T, Miyake H, Hara I, Fujisawa M. Expression of major heat shock proteins in prostate cancer: correlation with clinicopathological outcomes in patients undergoing radical prostatectomy. J Urol. 2007. 177:757–761.17. Oue T, Yoneda A, Uehara S, Yamanaka H, Fukuzawa M. Increased expression of the hedgehog signaling pathway in pediatric solid malignancies. J Pediatr Surg. 2010. 45:387–392.18. Bozkurt SU, Ayan E, Bolukbasi F, Elmaci I, Pamir N, Sav A. Immunohistochemical expression of SPARC is correlated with recurrence, survival and malignant potential in meningiomas. APMIS. 2009. 117:651–659.19. Koo JS, Jung W. Clinicopathlogic and immunohistochemical characteristics of triple negative invasive lobular carcinoma. Yonsei Med J. 2011. 52:89–97.20. Lauth M, Toftgard R. The Hedgehog pathway as a drug target in cancer therapy. Curr Opin Investig Drugs. 2007. 8:457–461.21. Bostrom AK, Moller C, Nilsson E, Elfving P, Axelson H, Johansson ME. Sarcomatoid conversion of clear cell renal cell carcinoma in relation to epithelial-to-mesenchymal transition. Hum Pathol. 2012. 43:708–719.22. Barnett P, Arnold RS, Mezencev R, Chung LW, Zayzafoon M, Odero-Marah V. Snail-mediated regulation of reactive oxygen species in ARCaP human prostate cancer cells. Biochem Biophys Res Commun. 2011. 404:34–39.23. Katz MD, Serrano MF, Humphrey PA, Grubb RL 3rd, Skolarus TA, Gao F, et al. The role of lymphovascular space invasion in renal cell carcinoma as a prognostic marker of survival after curative resection. Urol Oncol. 2011. 29:738–744.24. Xu QR, Zheng X, Zan XF, Yao YM, Yang W, Liu QG. Gli1 expression and its relationship with the expression of Shh, Vimentin and E-cadherin in human hepatocellular carcinoma. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2012. 28:536–539.25. Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009. 137:1478–1488.e8.26. Li X, Ma Q, Xu Q, Liu H, Lei J, Duan W, et al. SDF-1/CXCR4 signaling induces pancreatic cancer cell invasion and epithelial-mesenchymal transition in vitro through non-canonical activation of Hedgehog pathway. Cancer Lett. 2012. 322:169–176.27. Podhajcer OL, Benedetti L, Girotti MR, Prada F, Salvatierra E, Llera AS. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008. 27:523–537.28. Bedke J, Buse S, Pritsch M, Macher-Goeppinger S, Schirmacher P, Haferkamp A, et al. Perinephric and renal sinus fat infiltration in pT3a renal cell carcinoma: possible prognostic differences. BJU Int. 2009. 103:1349–1354.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Membrane Proteins Involved in Epithelial-Mesenchymal Transition and Tumor Invasion: Studies on TMPRSS4 and TM4SF5
- Role of the Epithelial-Mesenchymal Transition in Bladder Cancer: From Prognosis to Therapeutic Target
- The Role of Epithelial-mesenchymal Transition in the Gastroenterology
- Epithelial Mesenchymal Transition in Drug Resistance and Metastasis of Lung Cancer
- Consideration of EphA2 in relation to epithelial-mesenchymal transition in uterine endometrial cancer