Korean J Physiol Pharmacol.
2008 Aug;12(4):171-176. 10.4196/kjpp.2008.12.4.171.
Roles of ERK and NF-kappa B in Interleukin-8 Expression in Response to Heat Shock Protein 22 in Vascular Smooth Muscle Cells
- Affiliations
-
- 1Department of Pharmacology, School of Medicine, Pusan National University, Busan, Korea. koanhoi@pusan.ac.kr
- KMID: 1838355
- DOI: http://doi.org/10.4196/kjpp.2008.12.4.171
Abstract
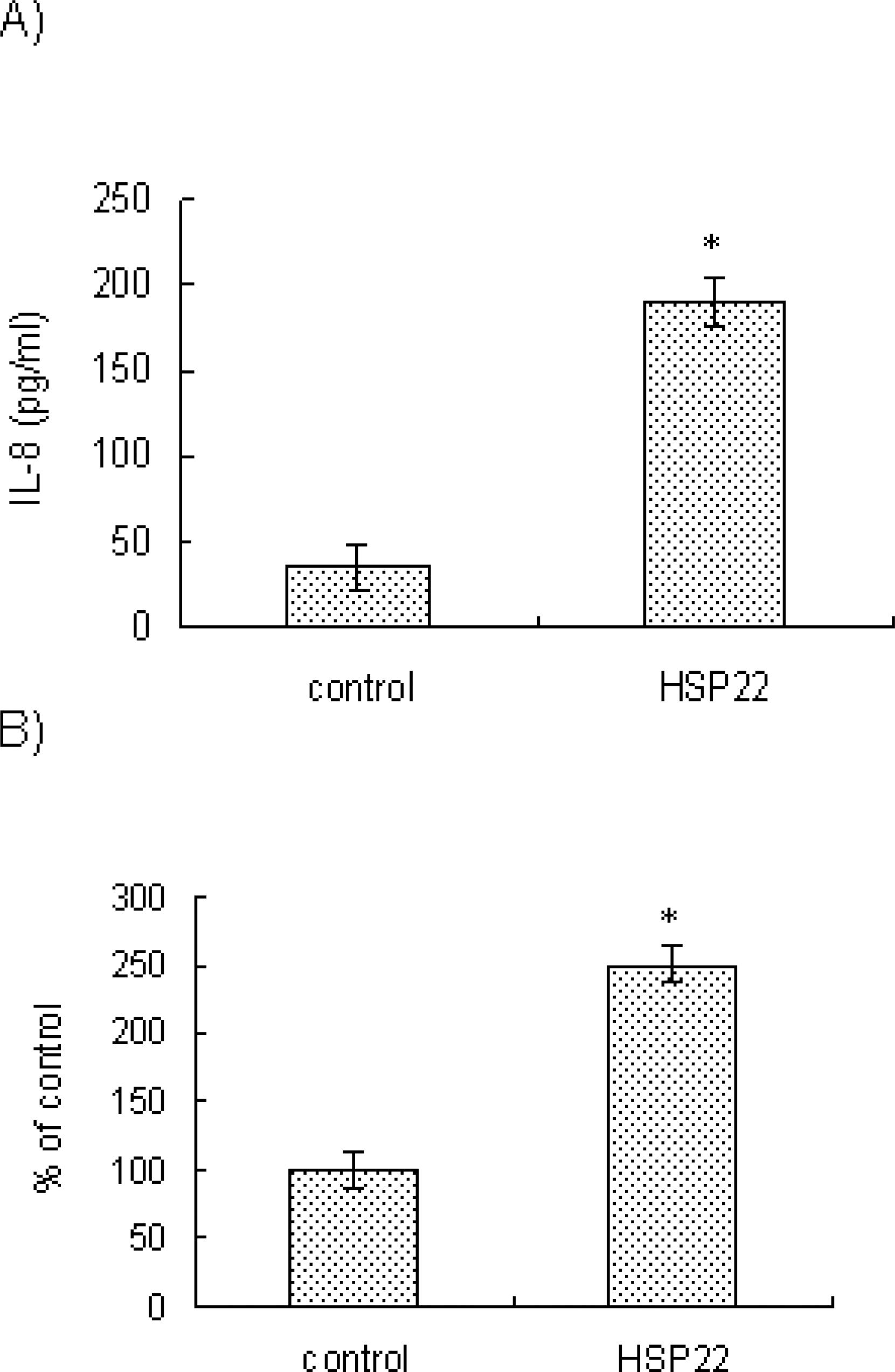
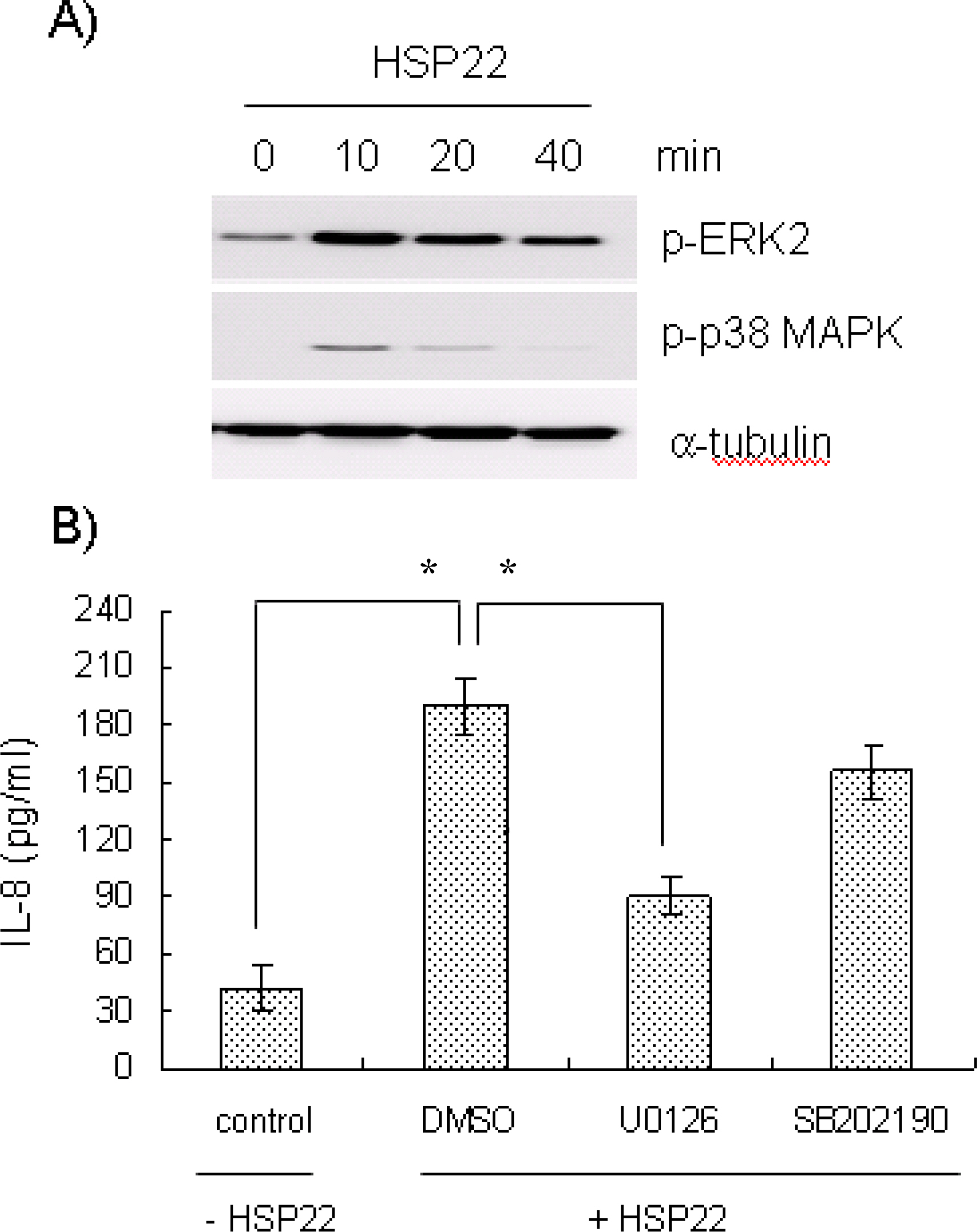
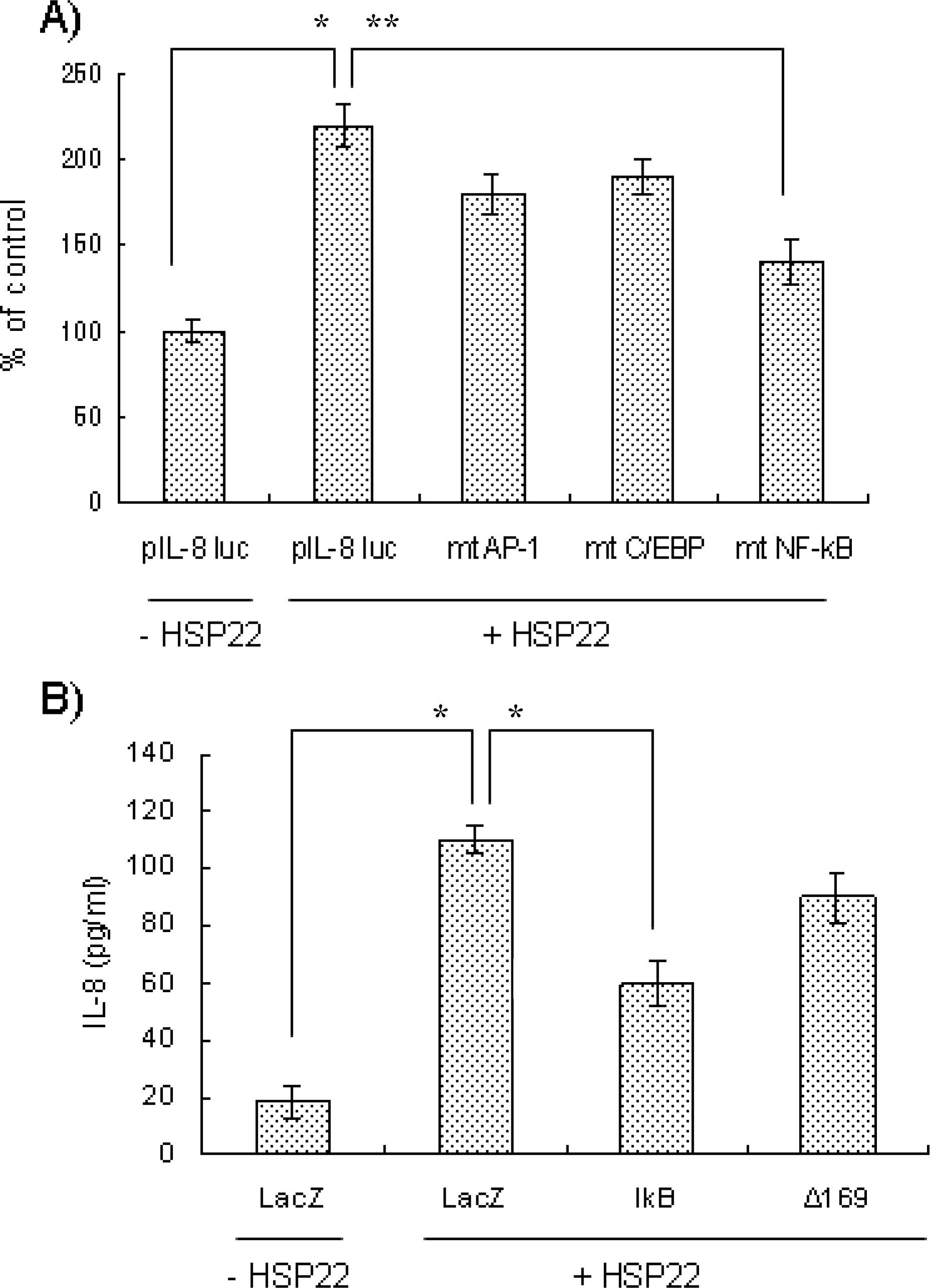
- Heat shock proteins (HSPs) serve as molecular chaperones and play a role in cell protection from damage in response to stress stimuli. The aim of this article is to investigate whether HSP22 affects IL-8 expression in vascular smooth muscle cells (VSMCs), and which cellular factors are involved in the HSP-mediated IL-8 induction in that cell type in terms of mitogen activated protein kinase (MAPK) and transcription element. Exposure of aortic smooth muscle cells (AoSMCs) to HSP22 not only enhanced IL-8 release but also induced IL-8 transcript via promoter activation. HSP22 activated ERK and p38 MAPK in AoSMCs. HSP22-induced IL-8 release was inhibited by U0126, but not by SB202190. A mutation in the IL-8 promoter region at the binding site of NF-kappa B, but not AP-1 or C/EBP, impaired promoter activation in response to HSP22. Delivery of I kappa B, but not dominant negative c-Jun, lowered HSP22-induced IL-8 release from AoSMCs. These results suggest that HSP22 induces IL-8 in VSMCs via ERK1/2, and that transcription factor NF-kB may be required for the HSP22-induced IL-8 up-regulation.
MeSH Terms
-
Binding Sites
Butadienes
Cytoprotection
Heat-Shock Proteins
Hot Temperature
I-kappa B Proteins
Imidazoles
Interleukin-8
Molecular Chaperones
Muscle, Smooth, Vascular
Myocytes, Smooth Muscle
NF-kappa B
Nitriles
p38 Mitogen-Activated Protein Kinases
Promoter Regions, Genetic
Protein Kinases
Proteins
Pyridines
Shock
Transcription Factor AP-1
Butadienes
Heat-Shock Proteins
I-kappa B Proteins
Imidazoles
Interleukin-8
Molecular Chaperones
NF-kappa B
Nitriles
Protein Kinases
Proteins
Pyridines
Transcription Factor AP-1
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
Aiyar N., Disa J., Ao Z., Ju H., Nerurkar S., Willette RN., Macphee CH., Johns DG., Douglas SA. Lysophosphatidylcholine induces inflammatory activation of human coronary artery smooth muscle cells. Mol Cell Biochem. 295:113–120. 2007.
ArticleAkira S., Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 4:499–511. 2004.
ArticleBoisvert WA., Santiago R., Curtiss LK., Terkeltaub RA. A leukocyte homologue of the IL-8 receptor CXCR-2 mediates the accumulation of macrophages in atherosclerotic lesions of LDL receptor-deficient mice. J Clin Invest. 101:353–363. 1998.
ArticleBrown PH., Chen TK., Birrer MJ. Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene. 9:791–799. 1994.de Jong WW., Leunissen JA., Voorter CE. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 10:103–126. 1993.Gerszten RE., Garcia-Zepeda EA., Lim YC., Yoshida M., Ding HA., Gimbrone MA., Jr. Luster A D., Luscinskas FW. Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 398:718–723. 1999.Ho FM., Kang HC., Lee ST., Chao Y., Chen YC., Huang LJ., Lin WW. The anti-inflammatory actions of LCY-2-CHO, a carbazole analogue, in vascular smooth muscle cells. Biochem Pharmacol. 74:298–308. 2007.
ArticleHuo Y., Weber C., Forlow SB., Sperandio M., Thatte J., Mack M., Jung S., Littman DR., Ley K. The chemokine KC, but not monocyte chemoattractant protein-1, triggers monocyte arrest on early atherosclerotic endothelium. J Clin Invest. 108:1307–1314. 2001.
ArticleInoue T., Komoda H., Nonaka M., Kameda M., Uchida T., Node K. Interleukin-8 as an independent predictor of long-term clinical outcome in patients with coronary artery disease. Int J Cardiol. 124:319–325. 2008.
ArticleJung YD., Fan F., McConkey DJ., Jean ME., Liu W., Reinmuth N., Stoeltzing O., Ahmad SA., Parikh AA., Mukaida N. Role of P38 MAPK, AP-1, and NF-kappaB in interleukin-1beta-induced IL-8 expression in human vascular smooth muscle cells. Cytokine. 18:206–213. 2002.Kawai T., Akira S. Toll-like receptor downstream signaling. Arthritis Res Ther. 7:12–19. 2005.MacRae TH. Structure and function of small heat shock/alpha-crystallin proteins: established concepts and emerging ideas. Cell Mol Life Sci. 57:899–913. 2000.Moreau M., Brocheriou I., Petit L., Ninio E., Chapman MJ., Rouis M. Interleukin-8 mediates down-regulation of tissue inhibitor of metalloproteinase-1 expression in cholesterol-loaded human macrophages: relevance to stability of atherosclerotic plaque. Circulation. 99:420–426. 1999.Perschinka H., Mayr M., Millonig G., Mayerl C., van der Zee R., Morrison SG., Morrison RP., Xu Q., Wick G. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler Thromb Vasc Biol. 23:1060–1065. 2003.
ArticlePeveri P., Walz A., Dewald B., Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 167:1547–1559. 1988.
ArticlePockley AG. Heat shock proteins as regulators of the immune response. Lancet. 362:469–476. 2003.
ArticlePockley AG., Georgiades A., Thulin T., de Faire U., Frostegard J. Serum heat shock protein 70 levels predict the development of atherosclerosis in subjects with established hypertension. Hypertension. 42:235–238. 2003.
ArticleRus HG., Vlaicu R., Niculescu F. Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis. 127:263–271. 1996.
ArticleSchroder JM., Christophers E. Secretion of novel and homologous neutrophil-activating peptides by LPS-stimulated human endothelial cells. J Immunol. 142:244–251. 1989.Srivastava P. Roles of heat-shock proteins in innate and adaptive immunity. Nat Rev Immunol. 2:185–194. 2002.
ArticleSteinberg D., Parthasarathy S., Carew TE., Khoo JC., Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 320:915–924. 1989.Tedgui A., Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 86:515–581. 2006.
ArticleWang JM., Sica A., Peri G., Walter S., Padura IM., Libby P., Ceska M., Lindley I., Colotta F. Mantovani A. Expression of monocyte chemotactic protein and interleukin-8 by cytokine-activated human vascular smooth muscle cells. Arterioscler Thromb. 11:1166–1174. 1991.Whitley D., Goldberg SP., Jordan WD. Heat shock proteins: a review of the molecular chaperones. J Vasc Surg. 29:748–751. 1999.
ArticleWick G., Perschinka H., Millonig G. Atherosclerosis as an autoimmune disease: an update. Trends Immunol. 22:665–669. 2001.
ArticleWu YM., Robinson DR., Kung HJ. Signal pathways in up-regulation of chemokines by tyrosine kinase MER/NYK in prostate cancer cells. Cancer Res. 64:7311–7320. 2004.
ArticleYang X., Coriolan D., Murthy V., Schultz K., Golenbock DT., Beasley D. Proinflammatory phenotype of vascular smooth muscle cells: role of efficient Toll-like receptor 4 signaling. Am J Physiol Heart Circ Physiol. 289:H1069–1076. 2005.
ArticleYang X., Murthy V., Schultz K., Tatro JB., Fitzgerald KA., Beasley D. Toll-like receptor 3 signaling evokes a proinflammatory and proliferative phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 291:H2334–2343. 2006.
ArticleYue TL., Wang X., Sung CP., Olson B., McKenna PJ., Gu JL., Feuerstein GZ. Interleukin-8. A mitogen and chemoattractant for vascular smooth muscle cells. Circ Res. 75:1–7. 1994.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fusobacterium nucleatum GroEL signaling via Toll-like receptor 4 in human microvascular endothelial cells
- Expression of Heat Shock Protein 70 in Vein Endothelial Cells Induced by Shear Stress
- Effect of Heat Shock on the Vascular Reactivity and Expression of Heat Shock Protein in an Animal Model of Type 2 Diabetes Mellitus (OLETF rat)
- Environmental factors regulating the expression of Porphyromonas gingivalis heat shock protein
- Lobaric Acid Inhibits VCAM-1 Expression in TNF-alpha-Stimulated Vascular Smooth Muscle Cells via Modulation of NF-kappaB and MAPK Signaling Pathways