Korean J Physiol Pharmacol.
2008 Aug;12(4):131-135. 10.4196/kjpp.2008.12.4.131.
Characterization of Ionic Currents in Human Neural Stem Cells
- Affiliations
-
- 1Department of Physiology, College of Medicine, Dankook University, Cheonan, Korea. ansil67@dku.edu
- 2Department of Physical Therapy, Gachon University of Medicine and Science Zucheon, Incheon, Korea.
- 3Department of Anatomy, Ajou University School of Medicine, Suwon, Korea.
- KMID: 1838349
- DOI: http://doi.org/10.4196/kjpp.2008.12.4.131
Abstract
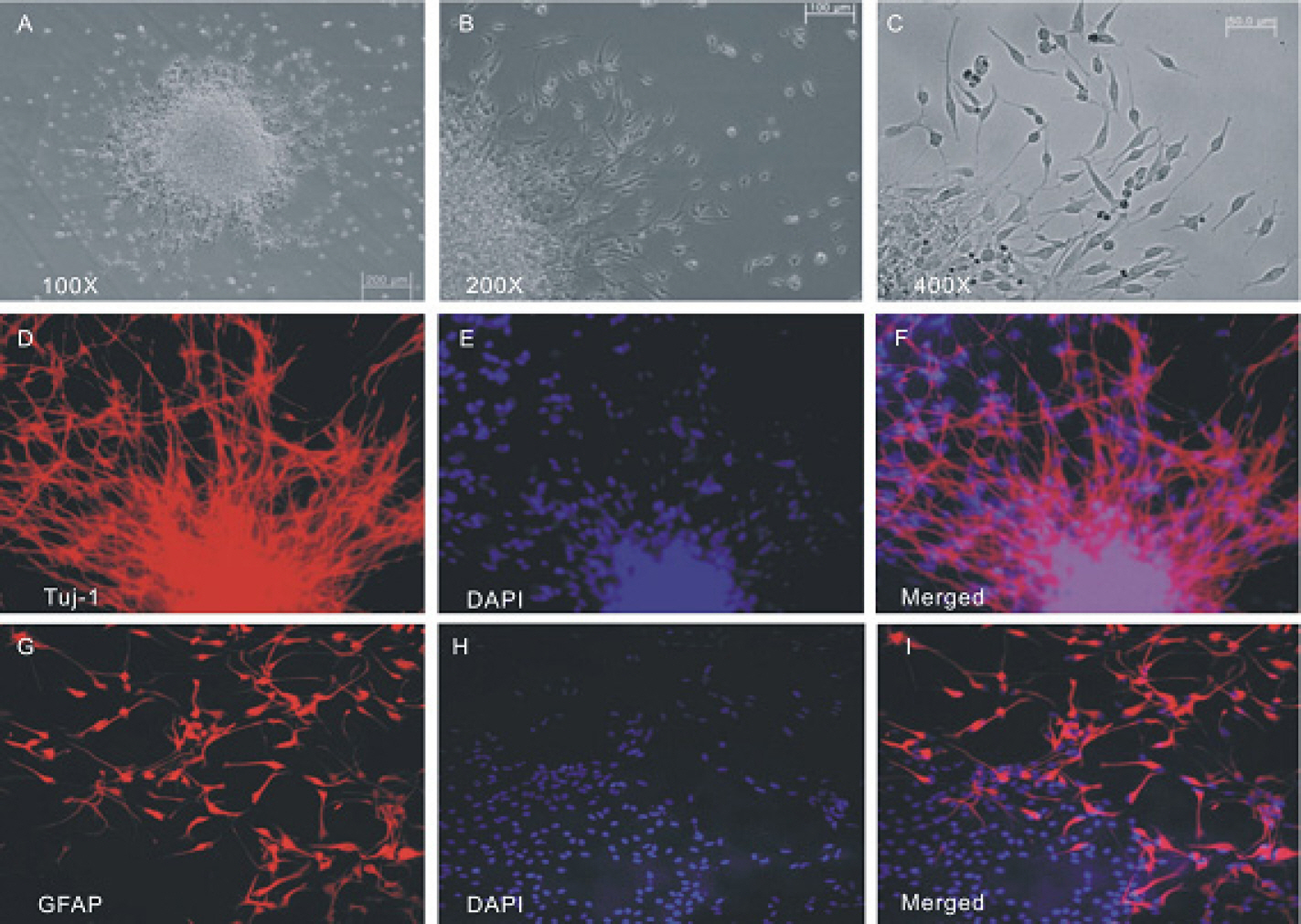
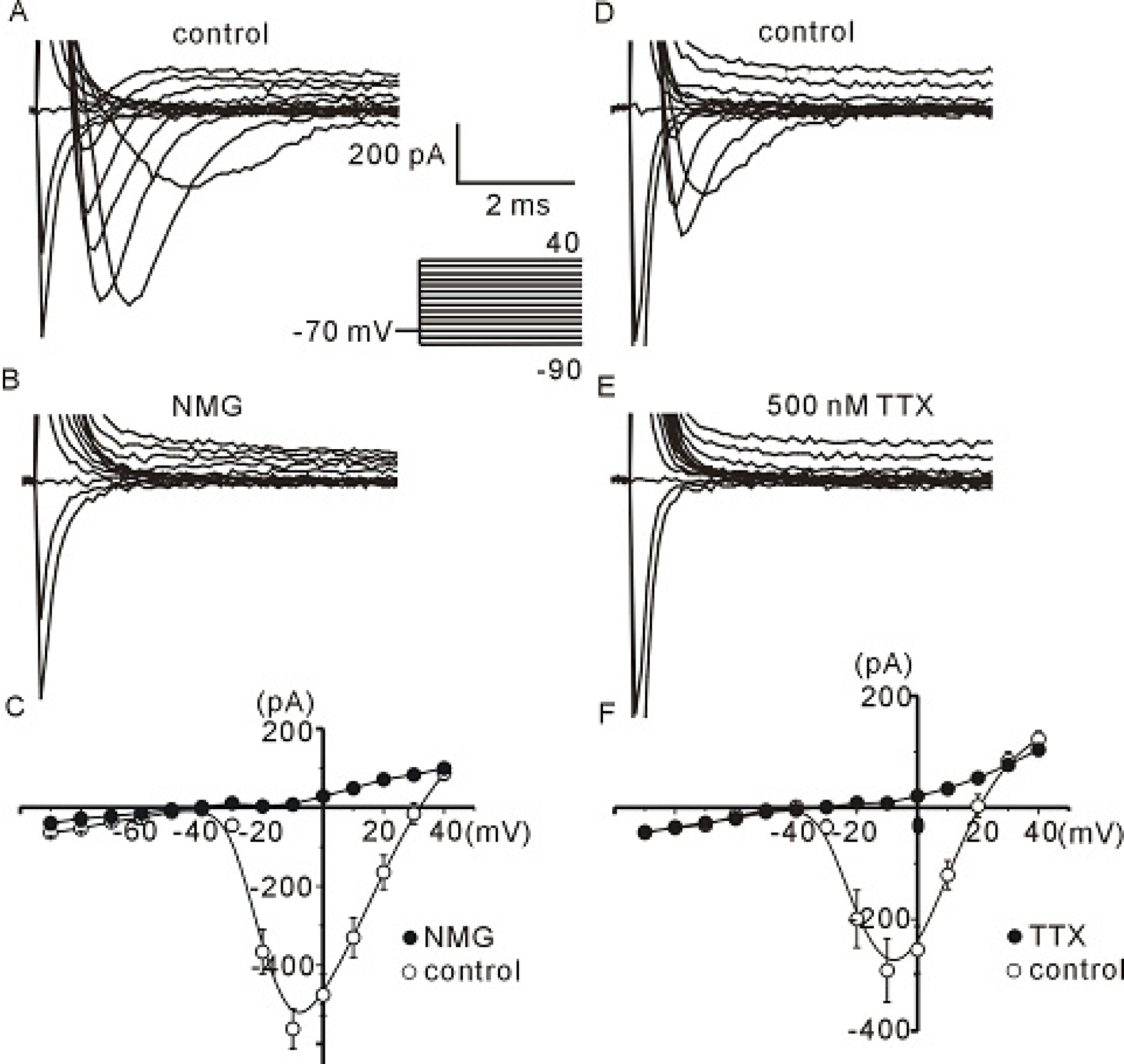
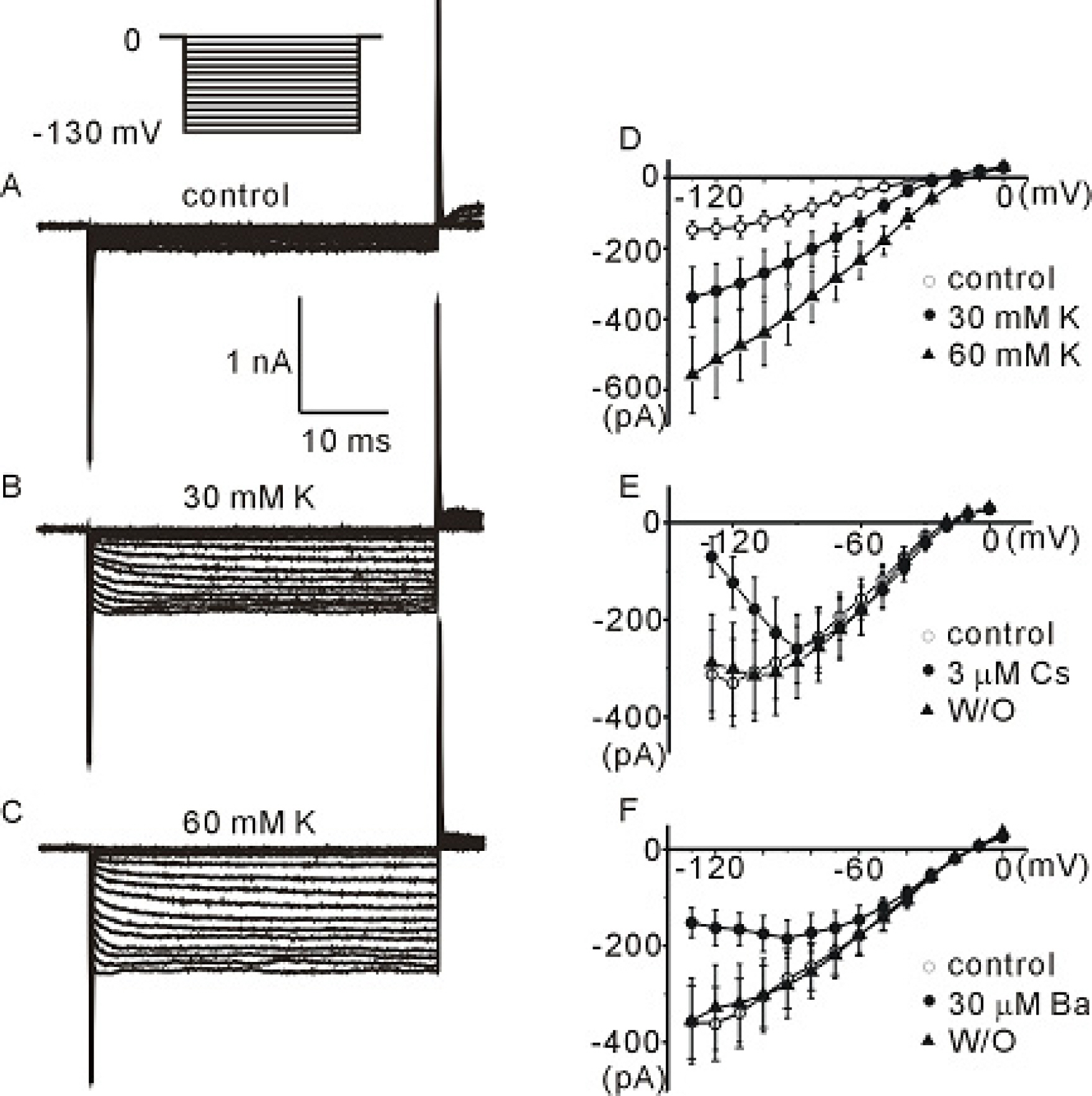
- The profile of membrane currents was investigated in differentiated neuronal cells derived from human neural stem cells (hNSCs) that were obtained from aborted fetal cortex. Whole-cell voltage clamp recording revealed at least 4 different currents: a tetrodotoxin (TTX)-sensitive Na+ current, a hyperpolarization-activated inward current, and A-type and delayed rectifier-type K+ outward currents. Both types of K+ outward currents were blocked by either 5 mM tetraethylammonium (TEA) or 5 mM 4-aminopyridine (4-AP). The hyperpolarization-activated current resembled the classical K+ inward current in that it exhibited a voltage-dependent block in the presence of external Ba2+ (30micrometer) or Cs+ (3micrometer). However, the reversal potentials did not match well with the predicted K+ equilibrium potentials, suggesting that it was not a classical K+ inward rectifier current. The other Na+ inward current resembled the classical Na+ current observed in pharmacological studies. The expression of these channels may contribute to generation and repolarization of action potential and might be regarded as functional markers for hNSCs-derived neurons.
Keyword
MeSH Terms
Figure
Reference
-
Akesson E., Piao JH., Samuelsson EB., Holmberg L., Kjaeldgaard A., Falci S., Sundstrom E., Seiger A. Long-term culture and neuronal survival after intraspinal transplantation of human spinal cord-derived neurospheres. Physiol Behav. 92:60–66. 2007.Artiani L., Bettiol E., Stillitano F., Mugelli A., Cerbai E., Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 25:1136–1144. 2007.Balasubramaniyan V., de Haas AH., Bakels R., Koper A., Boddeke HW., Copray JC. Functionally deficient neuronal differentiation of mouse embryonic neural stem cells in vitro. Neurosci Res. 49:261–265. 2004.
ArticleButt AM., Kalsi A. Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions. J Cell Mol Med. 10:33–44. 2006.
ArticleBuzańska L., Jurga M., Domańska-Janik K. Neuronal differentiation of human umbilical cord blood neural stem-like cell line. Neurodegener Dis. 3:19–26. 2006.
ArticleCarpenter MK., Inokuma MS., Denham J., Mujtaba T., Chiu CP., Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 172:383–397. 2001.
ArticleCho T., Bae JH., Choi HB., Kim SS., McLarnon JG., Suh-Kim H., Kim SU., Min CK. Human neural stem cells: electrophysiological properties of voltage-gated ion channels. Neuroreport. 13:1447–1452. 2002.
ArticleD'Ascenzo M., Piacentini R., Casalbore P., Budoni M., Pallini R., Azzena GB., Grassi C. Role of L-type Ca2+ channels in neural stem/progenitor cell differentiation. Eur J Neurosci. 23:935–944. 2006.Doupnik CA., Davidson N., Lester HA. The inward rectifier potassium channel family. Curr Opin Neurobiol. 5:268–277. 1995.
ArticleFlax JD., Aurora S., Yang C., Simonin C., Willis AM., Billinghurst LL., Jendoubi M., Sidman RL., Wolfe JH., Kim SU., Snyder EY. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 16:1033–1039. 1998.Flynn ER., McManus CA., Bradley KK., Koh SD., Hegarty TM., Horowitz B., Sanders KM. Inward rectifier potassium conductance regulates membrane potential of canine colonic smooth muscle. J Physiol. 518(Pt 1):247–256. 1999.
ArticleIlancheran S., Michalska A., Peh G., Wallace EM., Pera M., Manuelpillai U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 77:577–588. 2007.
ArticleJohnson MA., Weick JP., Pearce RA., Zhang SC. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci. 27:3069–3077. 2007.
ArticleLi GR., Sun H., Deng X., Lau CP. Characterization of ionic currents in human mesenchymal stem cells from bone marrow. Stem Cells. 23:371–382. 2005.
ArticleMareschi K., Novara M., Rustichelli D., Ferrero I., Guido D., Carbone E., Medico E., Madon E., Vercelli A., Fagioli F. Neural differentiation of human mesenchymal stem cells: Evidence for expression of neural markers and eag K+ channel types. Exp Hematol. 34:1563–1572. 2006.Moe MC., Westerlund U., Varghese M., Berg-Johnsen J., Svensson M., Langmoen LA. Development of neuronal networks from single stem cells harvested from the adult human brain. Neurosurgery. 56:1182–1188. 2005a.
ArticleMoe MC., Varghese M., Danilov AI., Westerlund U., Ramm-Pettersen J., Brundin L., Svensson M., Berg-Johnsen J., Langmoen IA. Multipotent progenitor cells from the adult human brain: neurophysiological differentiation to mature neurons. Brain. 128:2189–2199. 2005b.
ArticleMurata Y., Fujiwara Y., Kubo Y. Identification of a site involved in the block by extracellular Mg(2+) and Ba(2+) as well as permeation of K(+) in the Kir2.1 K(+) channel. J Physiol. 544:665–677. 2002.Nichols CG., Lopatin AN. Inward rectifier potassium channels. Ann Rev Physiol. 47:11–39. 1997.
ArticlePark KS., Jung KH., Kim SH., Kim KS., Choi MR., Kim Y., Chai YG. Functional expression of ion channels in mesenchymal stem cells derived from umbilical cord vein. Stem Cells. 25:2044–2052. 2007.
ArticlePiper DR., Mujtaba T., Rao MS., Lucero MT. Immunocytochemical and physiological characterization of a population of cultured human neural precursors. J Neurophysiol. 84:534–548. 2000.
ArticleSun W., Buzanska L., Domanska-Janik K., Salvi RJ., Stachowiak MK. Voltage-sensitive and ligand-gated channels in differentiating neural stem-like cells derived from the nonhematopoietic fraction of human umbilical cord blood. Stem Cells. 23:931–945. 2005.
ArticleThompson GA., Leyland ML., Ashmole I., Sutcliffe MJ., Stanfield PR. Residues beyond the selectivity filter of the K+ channel kir2.1 regulate permeation and block by external Rb+ and Cs+. J Physiol. 526:231–240. 2000.Weiss S., Reynolds BA., Vescovi AL., Morshead C., Craig CG., Van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 19:387–393. 1996.
ArticleWesterlund U., Moe MC., Varghese MM., Berg-Johnsen J., Ohlsson M., Langmoen IA., Svensson M. Stem cells from the adult human brain develop into functional neurons in culture. Exp Cell Res. 289:378–383. 2003.
ArticleZahanich I., Graf EM., Heubach JF., Hempel U., Boxberger S., Ravens U. Molecular and functional expression of voltage-operated calcium channels during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res. 20:1637–1646. 2005.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Propofol in Voltage-dependent Potassium Channels in Human Neurl Stem Cells
- Current Concepts of Stem Cell Therapy
- Effects of Fluoxetine on Membrane Potential and Ionic Currents in RINm5F Insulinoma Cells
- Clinical Applications of Neural Stem Cells for the Treatment of Peripheral Neuropathy
- A Simple Method for Generating Cerebral Organoids from Human Pluripotent Stem Cells