J Clin Neurol.
2005 Oct;1(2):148-158. 10.3988/jcn.2005.1.2.148.
Predicting the Long-Term Outcome after Subacute Stroke within the Middle Cerebral Artery Territory
- Affiliations
-
- 1Department of Neurology, Ajou University School of Medicine, Suwon, Korea. nmboy@unitel.co.kr
- KMID: 1808485
- DOI: http://doi.org/10.3988/jcn.2005.1.2.148
Abstract
- BACKGROUND AND PURPOSE
The National Institutes of Health Stroke Scale (NIHSS) score is known to be effective in predicting the likelihood of recovery after stroke. However, the baseline NIHSS score predicts long-term outcomes rather crudely because early changes in stroke scores may influence the stroke outcomes. Therefore, a precise prognostic algorithm or a cutoff point for predicting long-term outcomes based on data from serial NIHSS scores is needed.
METHODS
We serially assessed 437 patients with acute symptomatic ischemic stroke within the middle cerebral artery territory who presented with nonlacunar stroke and were followed-up for at least 6 months after symptom onset. The NIHSS score was serially checked at 0, 1, 3, 7, and 14 days after admission. In all patients, the Barthel index (BI) and the modified Rankin Scale (mRS) score were checked, with a poor outcome defined as any of the following endpoints: death, modified mRS score of >3, or BI of <60.
RESULTS
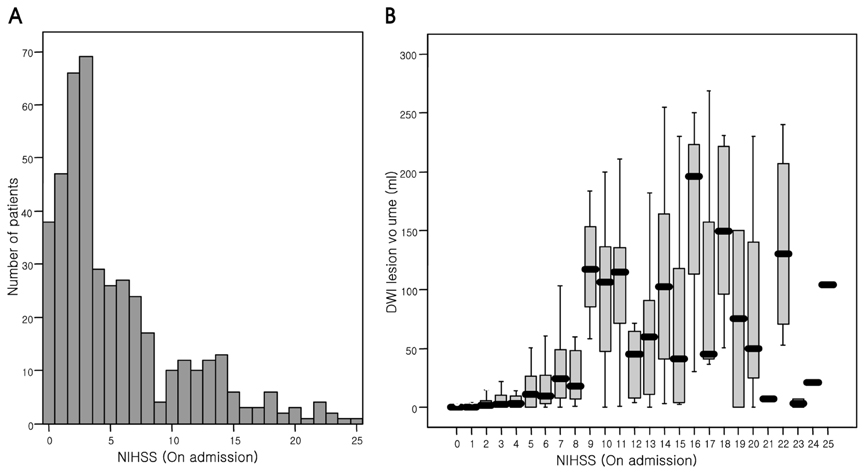
A marked neurological improvement or worsening (i.e., a change in the NIHSS score of at least 4) was seen in 13.5% or 5.5% of the patients, respectively, during the first 7 days after admission. About 25% of the 437 patients had poor long-term outcomes. Analysis of receiver operating characteristic curves showed that the NIHSS score at day 7 after admission was better for predicting poor long-term outcomes than was the baseline score (P=0.003). In addition, we analyzed the cutoff point of the 7th-day NIHSS score for predicting a poor outcome at 6 months after symptom onset. An NIHSS score of at least 6 at day 7 after admission predicted poor long-term outcomes with a sensitivity of 84% [95% confidence interval (CI), 76-90%], a specificity of 92% (95% CI, 88-94%), and positive and negative predictive values of 77% and 95%, respectively. A logistic regression analysis revealed that age, diffusion-weighted imaging lesion volume, stroke history, and 7th-day NIHSS score were independently associated with poor outcome. However, no score used in addition to the 7th-day NIHSS score improved the prediction of a poor outcome.
CONCLUSIONS
An NIHSS score of at least 6 on day 7 after admission accurately forecasts a poor long-term outcome after stroke. Our data may be helpful in predicting the long-term prognosis as well as in making decisions regarding novel therapeutic applications in subacute-stroke trials.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Current Status of Cell Therapies in Stroke
Oh Young Bang
Int J Stem Cells. 2009;2(1):35-44.
Reference
-
1. De Haan R, Horn J, Limburg M, Van Der Meulen J, Bossuyt P. A comparison of five stroke scales with measures of disability, handicap, and quality of life. Stroke. 1993. 24:1178–1181.
Article2. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995. 333:1581–1587.3. Fiorelli M, Alperovitch A, Argentino C, Sacchetti ML, Toni D, Sette G, et al. Prediction of long-term outcome in the early hours following acute ischemic stroke. Italian Acute Stroke Study Group. Arch Neurol. 1995. 52:250–255.
Article4. Muir KW, Weir CJ, Murray GD, Povey C, Lees KR. Comparison of neurological scales and scoring systems for acute stroke prognosis. Stroke. 1996. 27:1817–1820.
Article5. Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Acute stroke. Prognosis and a prediction of the effect of medical treatment on outcome and health care utilization. The Copenhagen Stroke Study. Neurology. 1997. 49:1335–1342.
Article6. Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC. German Stroke Study Collaboration. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. 2004. 35:158–162.
Article7. Braitman LE, Davidoff F. Predicting clinical states in individual patients. Ann Intern Med. 1996. 125:406–412.
Article8. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996. 15:361–387.
Article9. Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000. 55:565–569.
Article10. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005. 57:874–882.
Article11. Kwakkel G, Wagenaar RC, Kollen BJ, Lankhorst GJ. Predicting disability in stroke - a critical review of the literature. Age Ageing. 1996. 25:479–489.
Article12. Adams HP Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999. 53:126–131.
Article13. Bang OY, Lee PH, Joo SY, Lee JS, Joo IS, Huh K. Frequency and mechanisms of stroke recurrence after cryptogenic stroke. Ann Neurol. 2003. 54:227–234.
Article14. Brott TG, Haley EC Jr, Levy DE, Barsan W, Broderick J, Sheppard GL, et al. Urgent therapy for stroke. Part I. Pilot study of tissue plasminogen activator administered within 90 minutes. Stroke. 1992. 23:632–640.
Article15. Duncan PW, Jorgensen HS, Wade DT. Outcome measures in acute stroke trials: a systematic review and some recommendations to improve practice. Stroke. 2000. 31:1429–1438.16. Young FB, Lees KR, Weir CJ. Glycine Antagonist in Neuroprotection International Trial Steering Committee and Investigators. Strengthening acute stroke trials through optimal use of disability end points. Stroke. 2003. 34:2676–2680.
Article17. Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, et al. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002. 33:2243–2246.18. Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999. 30:1538–1541.
Article19. Counsell C, Dennis M, McDowall M, Warlow C. Predicting outcome after acute and subacute stroke: development and validation of new prognostic models. Stroke. 2002. 33:1041–1047.20. Tilling K, Sterne JA, Rudd AG, Glass TA, Wityk RJ, Wolfe CD. A new method for predicting recovery after stroke. Stroke. 2001. 32:2867–2873.
Article21. Baird AE, Dambrosia J, Janket S, Eichbaum Q, Chaves C, Silver B, et al. A three-item scale for the early prediction of stroke recovery. Lancet. 2001. 357:2095–2099.
Article22. Linfante I, Llinas RH, Schlaug G, Chaves C, Warach S, Caplan LR. Diffusion-weighted imaging and National Institutes of Health Stroke Scale in the acute phase of posterior-circulation stroke. Arch Neurol. 2001. 58:621–628.
Article23. Wityk RJ, Pessin MS, Kaplan RF, Caplan LR. Serial assessment of acute stroke using the NIH Stroke Scale. Stroke. 1994. 25:362–365.
Article24. Toni D, Fiorelli M, Bastianello S, Falcou A, Sette G, Ceschin V, et al. Acute ischemic strokes improving during the first 48 hours of onset: predictability, outcome, and possible mechanisms. A comparison with early deteriorating strokes. Stroke. 1997. 28:10–14.
Article25. Dorman PJ, Sandercock PA. Considerations in the design of clinical trials of neuroprotective therapy in acute stroke. Stroke. 1996. 27:1507–1515.
Article26. Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Survival and recurrence after first cerebral infarction: a population-based study in Rochester, Minnesota, 1975 through 1989. Neurology. 1998. 50:208–216.
Article27. Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke. 2000. 31:1062–1068.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lenticulostriate Artery Involvement is Predictive of Poor Outcomes in Superficial Middle Cerebral Artery Territory Infarction
- Acute Cerebral Infarction in Carbon Monoxide Poisoning
- Fibromuscular Dysplasia of the Distal Internal Carotid and Middle Cerebral Artery
- The Effect of Initial Serum Neuron-Specific Enolase Level on Clinical Outcome in Acute Carotid Artery Territory Infarction
- Middle Cerebral Artery Territory Infarction Caused by Giant Middle Cerebral Artery Dissecting Aneurysm