Cancer Res Treat.
2004 Feb;36(1):62-67.
A 21-day Schedule of Gemcitabine and Cisplatin Administration in the Treatment of Advanced Non-Small Cell Lung Carcinoma: a Phase II Study
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Dong-A University, Dong-A Cancer Center, Busan, Korea.
Abstract
- PURPOSE
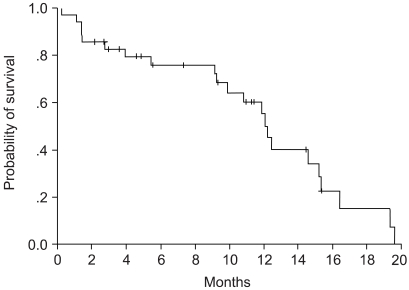
To evaluate the efficacy and toxicity of gemcitabine and cisplatin combination chemotherapy, we conducted a phase II study of this regimen in patients with advanced non-small cell lung carcinoma (NSCLC). MATERIALS AND METHODS: From June 2001 to August 2003, 36 chemotherapy- naive patients with stage IIIB or IV NSCLC were enrolled. The median age was 59 years (range, 42 to 75 years), and performance status was 0 or 1. Eleven patients had stage IIIB disease, and 25 patients had stage IV disease. 1, 000 mg/m2 of gemcitabine was administered on day 1 & 8, and 60 mg/m2 of cisplatin was administered on day 1. Each cycle was repeated every 21 days. RESULTS: Everyone subject who participated were assessable. A total of 160 cycles of chemotherapy were delivered, and the median number of chemotherapy courses was 3.5 (range, 2 to 9). Two patients (5.6%) achieved a complete response, and 14 patients (38.9%) achieved a partial response. The overall response rate was 44.5% (95% confidence interval [CI], 32.5 to 56.5%). The median follow-up duration was 9.3 months. The median time to disease progression was 8.6 months (95% CI 7.4 to 9.9 months), and median survival time was 12.2 months (95% CI, 10.5 to 12.9 months). Grade 3/4 neutropenia occurred in 9 patients (25.0%), neutropenic fever occurred in 3 patients (8.3%), and grade 3/4 thrombocytopenia occurred in 7 patients (19.5%). Mild forms of non-hematologic toxicities, such as nausea, vomiting or skin reactions, were observed. CONCLUSION: The combination of gemcitabine and cisplatin in a 21-day schedule is an effective regimen for patients with NSCLC in its advanced stages.
MeSH Terms
Figure
Reference
-
1. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer. A meta-analysis using updated data on individual patients from 52 randomized clinical trials. BMJ. 1995; 311:899–909. PMID: 7580546.2. Perng RP, Chen YM, Ming-Liu J, Tsai CM, Lin WC, Yang KY, Whang-Peng J. Gemcitabine versus the combination of cisplatin and etoposide in patients with inoperable non-small-cell lung cancer in a phase II randomized study. J Clin Oncol. 1997; 15:2097–2102. PMID: 9164223.
Article3. Vansteenkiste JF, Vandebroek JE, Nackaerts KL, Weynants P, Valcke YJ, Verresen DA, Devogelaere RC, Marien SA, Humblet YP, Dams NL. Leuven Lung Cancer Group. Clinical-benefit response in advanced non-small-cell lung cancer: A multicenter prospective randomized phase III study of single agent gemcitabine versus cisplatin-vindesine. Ann Oncol. 2001; 12:1221–1230. PMID: 11697832.4. Le Chevalier T, Brisgand D, Pujol JL, Douillard JY, Monnier A, Riviere A, Chomy P, Le Groumellec A, Ruffie P, Gottfried M, Gaspard MH, Chevreau C, Alberola V, Cigolari S, Besson F, Martinez A, Besenval M, Berthaud P, Tursz T. Results of a randomized study comparing combination of navelbine-cisplatin to combination of vindesine-cisplatin and to navelbine alone in 612 patients with inoperable non-small cell lung cancer. Bull Cancer. 1996; 83:385–394. PMID: 8680091.5. Bonomi P, Kim K, Fairclough D, Cella D, Kugler J, Rowinsky E, Jiroutek M, Johnson D. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000; 18:623–631. PMID: 10653877.
Article6. Cardenal F, Lopez-Cabrerizo MP, Anton A, Alberola V, Massuti B, Carrato A, Barneto I, Lomas M, Garcia M, Lianes P, Montalar J, Vadell C, Gonzalez-Larriba JL, Nguyen B, Artal A, Rosell R. Randomized phase III study of gemcitabine-cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 1999; 17:12–18. PMID: 10458212.
Article7. O'Rourke TJ, Brown TD, Havlin K, Kuhn JG, Craig JB, Burris HA, Satterlee WG, Tarassoff PG, Von Hoff DD. Phase I clinical trial of gemcitabine given as an intravenous bolus on 5 consecutive days. Eur J Cancer. 1994; 30:417–418. PMID: 8204374.8. Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W, Raber MN, et al. A phase I clinical, plasma and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991; 9:491–498. PMID: 1999720.
Article9. Abratt RP, Bezwoda WR, Falkson G, Goedhals L, Hacking D, Rugg TA. Efficacy and safety profile of gemcitabine in non-small-cell lung cancer: a phase II study. J Clin Oncol. 1994; 12:1535–1540. PMID: 8040664.
Article10. Anderson H, Lund B, Bach F, Thatcher N, Walling J, Hansen HH. Single-agent activity of weekly gemcitabine in advanced non-small-cell lung cancer: a phase II study. J Clin Oncol. 1994; 12:1821–1826. PMID: 8083706.
Article11. Gatzemeier U, Shepherd FA, Le Chevalier T, Weynants P, Cottier B, Groen HJ, Rosso R, Mattson K, Cortes-Funes H, Tonato M, Burkes RL, Gottfried M, Voi M. Activity of gemcitabine in patients with non-small cell lung cancer: a multicentre, extended phase II study. Eur J Cancer. 1996; 32:243–248. PMID: 8664035.
Article12. Peters GJ, Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Braakhuis BJ. Interaction between cisplatin and gemcitabine in vitro and in vivo. Semin Oncol. 1995; 22:72–79. PMID: 7481849.13. Poplin EA, Corbett T, Flaherty L, Tarasoff P, Redman BG, Valdivieso M, Baker L. Difluorodeoxycytidine (dFdC)--gemcitabine: a phase I study. Invest New Drugs. 1992; 10:165–170. PMID: 1428726.14. Abratt RP, Hacking DJ, Goedhals L, Bezwoda WR. Weekly gemcitabine and monthly cisplatin for advanced non-small cell lung carcinoma. Semin Oncol. 1997; 24(3 Suppl 8):S8-18–S8-23. PMID: 9207311.15. Crino L, Scagliotti G, Marangolo M, Figoli F, Clerici M, De Marinis F, Salvati F, Cruciani G, Dogliotti L, Pucci F, Paccagnella A, Adamo V, Altavilla G, Incoronato P, Trippetti M, Mosconi AM, Santucci A, Sorbolini S, Oliva C, Tonato M. Cisplatin-gemcitabine combination in advanced non-small-cell lung cancer: a phase II study. J Clin Oncol. 1997; 15:297–303. PMID: 8996156.16. Steward WP, Dunlop DJ, Cameron C, Talbot DC, Kleisbauer JP, Thomas P, Guerin JC, Perol M, Sanson C, Dabouis G, et al. Combination chemotherapy with gemcitabine and cisplatin in the treatment of advanced non-small cell lung cancer: preliminary results of an ongoing phase I/II study. Anticancer Drugs. 1995; 6:33–37. PMID: 8718423.
Article17. Shepherd FA, Cormier Y, Burkes R, Evans WK, Goss G, Klimo P, Feld R, Taylor M. Phase II trial of gemcitabine and weekly cisplatin for advanced non-small cell lung cancer. Semin Oncol. 1997; 24(3 Suppl 8):S8-27–S8-30. PMID: 9207313.18. Abratt RP, Sandler A, Crino L, Steward WP, Shepherd FA, Green MR, Nguyen B, Peters GJ. Combined cisplatin and gemcitabine for non-small cell lung cancer: influence of scheduling on toxicity and drug delivery. Semin Oncol. 1998; 25(4 Suppl 9):35–43. PMID: 9728583.19. Shepherd FA, Abratt R, Crino L, Green M, Sandler A, Steward W, Iglesias J, Anglin G. The influence of gemcitabine and cisplatin schedule on response and survival in advanced non-small cell lung cancer. Lung Cancer. 2000; 30:117–125. PMID: 11086205.
Article20. Smit EF, van Meerbeeck JP, Lianes P, Debruyne C, Legrand C, Schramel F, Smit H, Gaafar R, Biesma B, Manegold C, Neymark N, Giaccone G. European Organization for Research and Treatment of Cancer Lung Cancer Group. Three-arm randomized study of two cisplatin-based regimens and paclitaxel plus gemcitabine in advanced non-small-cell lung cancer: a phase III trial of the European Organization for Research and Treatment of Cancer Lung Cancer Group--EORTC 08975. J Clin Oncol. 2003; 21:3909–3917. PMID: 14581415.21. Scagliotti GV, De Marinis F, Rinaldi M, Crino L, Gridelli C, Ricci S, Matano E, Boni C, Marangolo M, Failla G, Altavilla G, Adamo V, Ceribelli A, Clerici M, Di Costanzo F, Frontini L, Tonato M. Italian Lung Cancer Project. Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer. J Clin Oncol. 2002; 20:4285–4291. PMID: 12409326.22. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH. Eastern Cooperative Oncology Group. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002; 346:92–98. PMID: 11784875.
Article23. Lund B, Kristjansen PE, Hansen HH. Clinical and preclinical activity of 2',2'-difluorodeoxycytidine (gemcitabine). Cancer Treat Rev. 1993; 19:45–55. PMID: 8431926.24. Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2',2'-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991; 51:6110–6117. PMID: 1718594.25. Park JW, Park HY, Park YB, Kang JW, Kim SH, Lee GL, Kim BS, Roh YH. A phase II study with gemcitabine and carboplatin in patients with advanced non-small cell lung cancer. Cancer Res Treat. 2002; 34:23–27.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Three-Weekly Gemcitabine Plus Cisplatin Chemotherapy in Patients with Locally Advanced or Metastatic Non-small-cell Lung Cancer: Phase II Study of the Korean Association for the Study of Lung Cancer
- 3-week-scheduled combination chemotherapy of gemcitabine and cisplatin in patients with advanced NSCLC
- Gemcitabine/Cisplatin Combination Chemotherapy in Advanced non-Small Cell lung Cancer
- A Phase II Study of Vinorelbine, Mitomycin C and Cisplatin Chemotherapy for Advanced Non-Small Cell Lung Cancer
- A Phase II Study of Combination Chemotherapy with Gemcitabine, 5-fluorouracil, and Cisplatin for Advanced Pancreatic Cancer