Cancer Res Treat.
2012 Dec;44(4):235-241.
Outcomes of Third-Line Docetaxel-Based Chemotherapy in Advanced Gastric Cancer Who Failed Previous Oxaliplatin-Based and Irinotecan-Based Chemotherapies
- Affiliations
-
- 1Department of Internal Medicine, Gyeongsang National University School of Medicine, Jinju, Korea.
- 2Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea. hematoonco@naver.com
- 3Department of Radiation Oncology, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Ilsan Hospital, Goyang, Korea.
- 6Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- 7Department of Internal Medicine, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 8Department of Internal Medicine, Institute of Health Science, Gyeongsang National University School of Medicine, Jinju, Korea.
Abstract
- PURPOSE
Little is known about outcomes in the use of third-line chemotherapy in cases of advanced gastric cancer (AGC). The primary aim of this retrospective study was to evaluate outcomes of docetaxel-based chemotherapy in patients with AGC that progressed after both oxaliplatin-based and irinotecan-based regimens.
MATERIALS AND METHODS
Eligible patients were those with AGC who had previous chemotherapy including fluoropyrimidine and oxaliplatin as well as fluoropyrimidine and irinotecan and who received subsequent docetaxel-based chemotherapy. Thirty-five patients were retrospectively recruited from 5 medical centers in Korea. Patients received either weekly or 3 weekly with docetaxel +/- cisplatin.
RESULTS
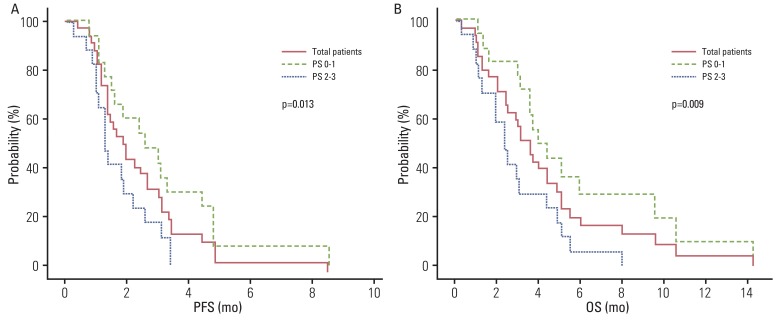
Thirty-one out of 35 patients were evaluated for treatment response. A total of 94 cycles of chemotherapy (median, 2; range, 1 to 7) were administered. The overall response rate was 14.3%, and the disease control rate was 45.7%. The median progression-free survival (PFS) was 1.9 months (95% confidence interval [CI], 1.1 to 2.7 months). The median overall survival (OS) was 3.6 months (95% CI, 2.8 to 4.4 months). PFS and OS were significantly prolonged in patients of the Eastern Cooperative Oncology Group, with performance status of 0 or 1 in multivariate analysis (PFS: hazard ratio[HR], 0.411; 95% CI, 0.195 to 0.868; p=0.020 and OS: HR, 0.390; 95% CI, 0.184 to 0.826; p=0.014, respectively). Four of the 35 patients enrolled in the study died due to infection associated with neutropenia.
CONCLUSION
Our findings suggest that salvage docetaxel-based chemotherapy is a feasible treatment option for AGC patients with good performance status (PS), whereas chemotherapy for patients with poor PS (PS< or =2) should be undertaken with caution for those who previously failed oxaliplatin- and irinotecan-based regimens.
Keyword
MeSH Terms
Figure
Reference
-
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90. PMID: 21296855.
Article2. Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, et al. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009; 24:995–1003. PMID: 19949651.
Article3. Kong SH, Park DJ, Lee HJ, Jung HC, Lee KU, Choe KJ, et al. Clinicopathologic features of asymptomatic gastric adenocarcinoma patients in Korea. Jpn J Clin Oncol. 2004; 34:1–7. PMID: 15020656.
Article4. Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997; 8:163–168. PMID: 9093725.
Article5. Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer. 1995; 71:587–591. PMID: 7533517.
Article6. Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010; (3):CD004064. PMID: 20238327.
Article7. Wohrer SS, Raderer M, Hejna M. Palliative chemotherapy for advanced gastric cancer. Ann Oncol. 2004; 15:1585–1595. PMID: 15520058.8. Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012; 30:1513–1518. PMID: 22412140.
Article9. Kang H, Kauh JS. Chemotherapy in the treatment of metastatic gastric cancer: is there a global standard? Curr Treat Options Oncol. 2011; 12:96–106. PMID: 21274667.
Article10. Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol. 2006; 24:2903–2909. PMID: 16782930.
Article11. Jeon EK, Hong SH, Kim TH, Jung SE, Park JC, Won HS, et al. Modified FOLFIRI as second-line chemotherapy after failure of modified FOLFOX-4 in advanced gastric cancer. Cancer Res Treat. 2011; 43:148–153. PMID: 22022291.
Article12. Lee HH, Hur H, Kim SH, Park AR, Kim W, Jeon HM. Outcomes of modified FOLFOX-6 as first line treatment in patients with advanced gastric cancer in a single institution: retrospective analysis. Cancer Res Treat. 2010; 42:18–23. PMID: 20369047.
Article13. Kim SH, Lee GW, Go SI, Cho SH, Kim HJ, Kim HG, et al. A phase II study of irinotecan, continuous 5-fluorouracil, and leucovorin (FOLFIRI) combination chemotherapy for patients with recurrent or metastatic gastric cancer previously treated with a fluoropyrimidine-based regimen. Am J Clin Oncol. 2010; 33:572–576. PMID: 20042971.
Article14. Kim BG, Oh SY, Kwon HC, Lee S, Lee DM, Kim SG, et al. A phase II study of irinotecan with biweekly, low dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFIRI) as first line therapy for patients with recurrent or metastatic gastric cancer. Am J Clin Oncol. 2010; 33:246–250. PMID: 19770628.
Article15. Khokhar NZ, Jiang Y, Benson AB 3rd, Ajani JA, Mulcahy MF. Refining docetaxel-containing therapy for gastric cancer. Gastrointest Cancer Res. 2011; 4:96–105. PMID: 22043325.16. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247. PMID: 19097774.
Article17. Moon YW, Rha SY, Jeung HC, Kim C, Hong MH, Chang H, et al. Outcomes of multiple salvage chemotherapy for advanced gastric cancer: implications for clinical practice and trial design. Cancer Chemother Pharmacol. 2010; 66:797–805. PMID: 20221831.
Article18. Shin SJ, Jeung HC, Ahn JB, Choi HJ, Cho BC, Rha SY, et al. Capecitabine and doxorubicin combination chemotherapy as salvage therapy in pretreated advanced gastric cancer. Cancer Chemother Pharmacol. 2008; 61:157–165. PMID: 17426971.
Article19. Shim HJ, Yun JY, Hwang JE, Bae WK, Cho SH, Chung IJ. Prognostic factor analysis of third-line chemotherapy in patients with advanced gastric cancer. Gastric Cancer. 2011; 14:249–256. PMID: 21431297.
Article20. Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004; 22:1209–1214. PMID: 15051767.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetic Polymorphism of GSTP1: Prediction of Clinical Outcome to Oxaliplatin/5-FU-based Chemotherapy in Advanced Gastric Cancer
- Irinotecan Monotherapy Versus Irinotecan-Based Combination as Second-Line Chemotherapy in Advanced Gastric Cancer: A Meta-Analysis
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?
- Docetaxel and cisplatin combination chemotherapy for advanced gastric cancer failed to 5-fluorouracil-based chemotherapy
- Chemotherapy of Advanced Gastric Cancer