Cancer Res Treat.
2011 Mar;43(1):56-66.
Alpha-Type 1 Polarized Dendritic Cells Loaded with Apoptotic Allogeneic Breast Cancer Cells Can Induce Potent Cytotoxic T Lymphocytes against Breast Cancer
- Affiliations
-
- 1Department of Surgery, Chonnam National University Hwasun Hospital, Chonnam National University School of Medicine, Hwasun, Korea.
- 2Department of Hematology-Oncology, Chonnam National University Hwasun Hospital, Chonnam National University School of Medicine, Hwasun, Korea. drjejung@chonnam.ac.kr
- 3Research Center for Cancer Immunotherapy, Chonnam National University Hwasun Hospital, Chonnam National University School of Medicine, Hwasun, Korea.
- 4The Brain Korea 21 Project, Center for Biomedical Human Resources at Chonnam National University, Gwangju, Korea.
Abstract
- PURPOSE
Various tumor antigens can be loaded onto dendritic cells (DCs) to induce a potent cytotoxic T lymphocyte (CTL) response in DC-based immunotherapy against breast cancer. However, in the clinical setting, obtaining a sufficient number of autologous tumor cells as a source of tumor antigens is a laborious process. We therefore investigated the feasibility of immunotherapy using breast-cancer-specific CTLs generated in vitro by use of alpha-type 1 polarized DCs (alpha DC1s) loaded with ultraviolet B-irradiated cells of the breast cancer cell line MCF-7.
MATERIALS AND METHODS
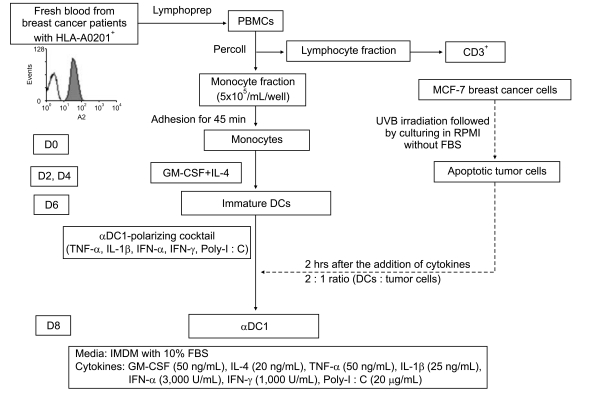
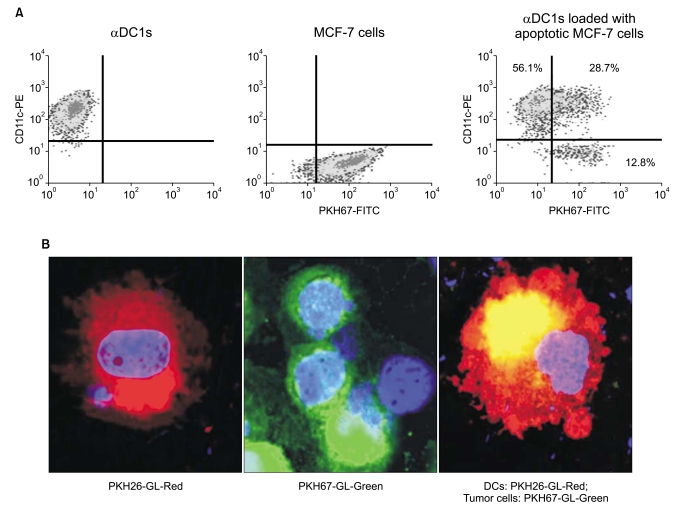
alphaDC1s were induced by loading allogeneic tumor antigen generated from the MCF-7 UVB-irradiated breast cancer cell line. Antigen-pulsed alphaDC1s were evaluated by morphological and functional assays, and the breast-cancer-specific CTL response was analyzed by cytotoxic assay.
RESULTS
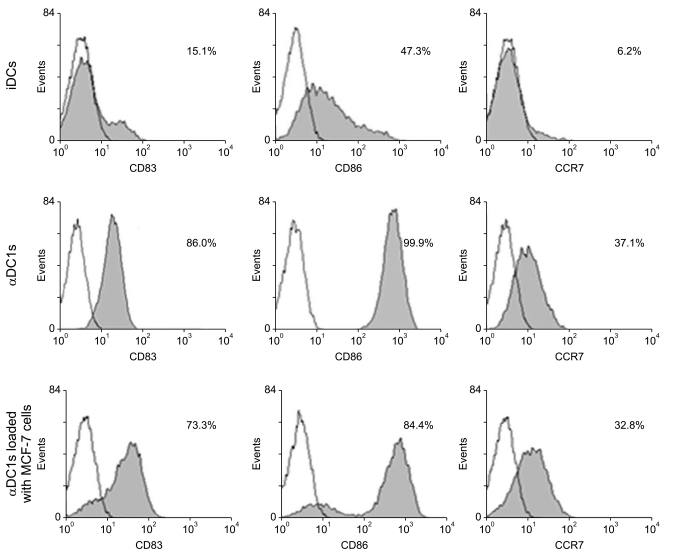
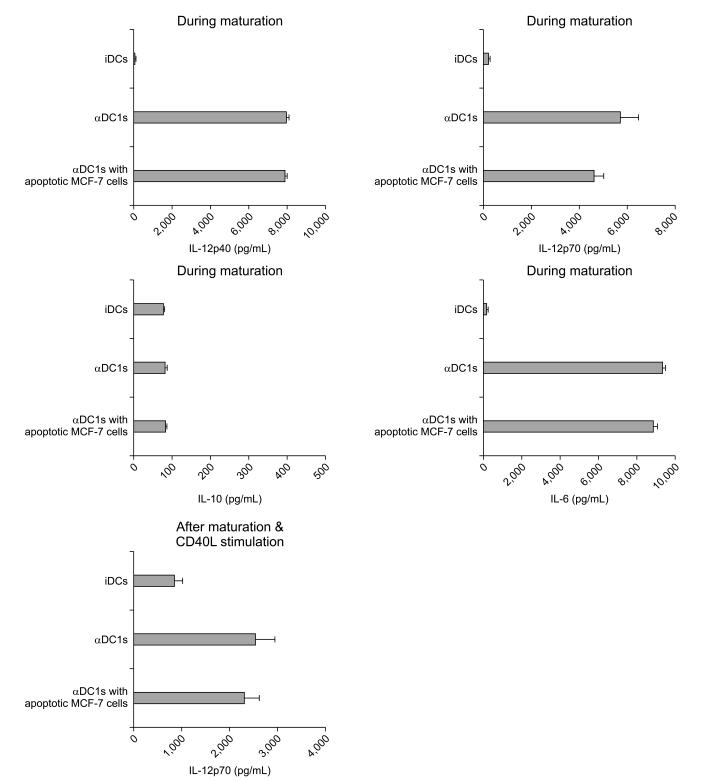
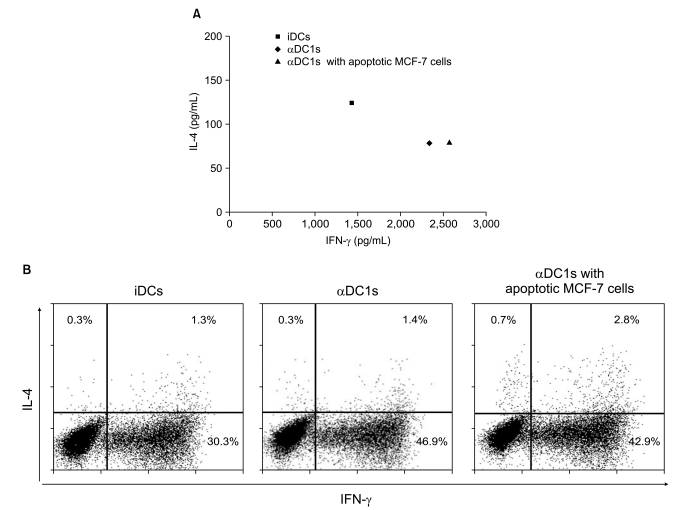
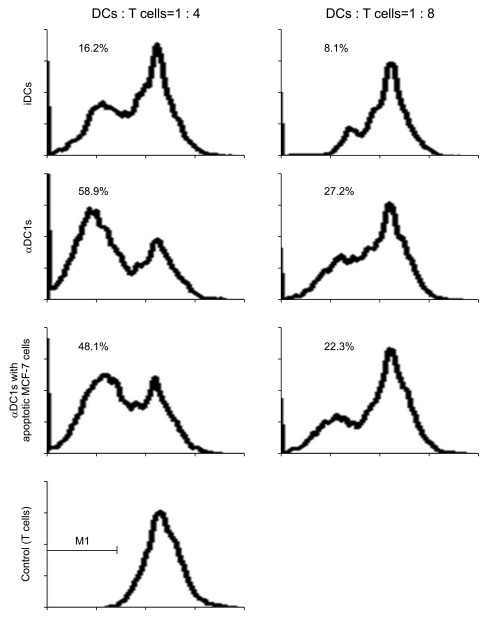
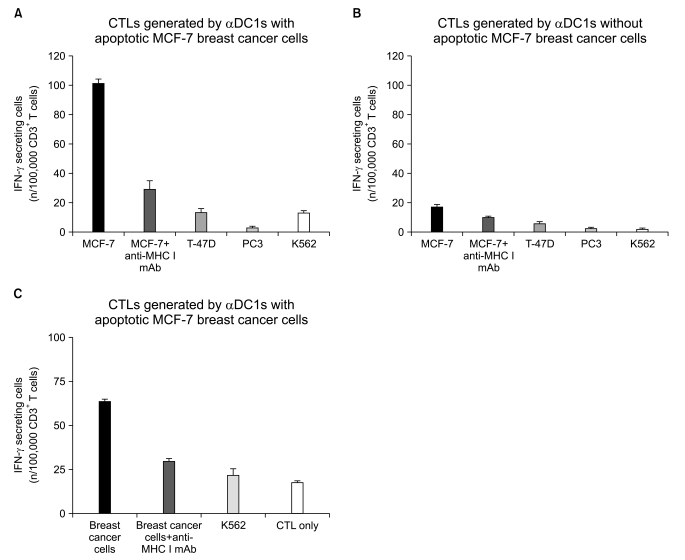
The alphaDC1s significantly increased the expression of several molecules related to DC maturation without differences according to whether the alphaDC1s were loaded with tumor antigens. The alphaDC1s showed a high production of interleukin-12 both during maturation and after subsequent stimulation with CD40L, which was not significantly affected by loading with tumor antigens. Breast-cancer-specific CTLs against autologous breast cancer cells were successfully induced by alphaDC1s loaded with apoptotic MCF-7 cells.
CONCLUSION
Autologous DCs loaded with an allogeneic breast cancer cell line can generate potent breast-cancer-specific CTL responses. This may be a practical method for cellular immunotherapy in patients with breast cancer.
MeSH Terms
Figure
Reference
-
1. Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003; 8:541–552. PMID: 14657533.
Article2. Anderson KS. Tumor vaccines for breast cancer. Cancer Invest. 2009; 27:361–368. PMID: 19358018.
Article3. Curigliano G, Spitaleri G, Dettori M, Locatelli M, Scarano E, Goldhirsch A. Vaccine immunotherapy in breast cancer treatment: promising, but still early. Expert Rev Anticancer Ther. 2007; 7:1225–1241. PMID: 17892423.
Article4. Chaudhuri S, Cariappa A, Tang M, Bell D, Haber DA, Isselbacher KJ, et al. Genetic susceptibility to breast cancer: HLA DQB*03032 and HLA DRB1*11 may represent protective alleles. Proc Natl Acad Sci U S A. 2000; 97:11451–11454. PMID: 11027344.
Article5. Disis ML, Calenoff E, McLaughlin G, Murphy AE, Chen W, Groner B, et al. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994; 54:16–20. PMID: 7505195.6. Jerome KR, Domenech N, Finn OJ. Tumor-specific cytotoxic T cell clones from patients with breast and pancreatic adenocarcinoma recognize EBV-immortalized B cells transfected with polymorphic epithelial mucin complementary DNA. J Immunol. 1993; 151:1654–1662. PMID: 8393050.7. Carella AM, Beltrami G, Corsetti MT, Nati S, Musto P, Scalzulli P, et al. Reduced intensity conditioning for allograft after cytoreductive autograft in metastatic breast cancer. Lancet. 2005; 366:318–320. PMID: 16039336.
Article8. Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998; 392:245–252. PMID: 9521319.
Article9. Reid DC. Dendritic cells and immunotherapy for malignant disease. Br J Haematol. 2001; 112:874–887. PMID: 11298582.
Article10. Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, et al. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004; 64:5934–5937. PMID: 15342370.11. Lee JJ, Foon KA, Mailliard RB, Muthuswamy R, Kalinski P. Type 1-polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. J Leukoc Biol. 2008; 84:319–325. PMID: 18426971.
Article12. Gilewski T, Adluri S, Ragupathi G, Zhang S, Yao TJ, Panageas K, et al. Vaccination of high-risk breast cancer patients with mucin-1 (MUC1) keyhole limpet hemocyanin conjugate plus QS-21. Clin Cancer Res. 2000; 6:1693–1701. PMID: 10815887.13. Murray JL, Gillogly ME, Przepiorka D, Brewer H, Ibrahim NK, Booser DJ, et al. Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTLs by HER-2 peptide E75 (369-377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2002; 8:3407–3418. PMID: 12429628.14. Parkhurst MR, Riley JP, Igarashi T, Li Y, Robbins PF, Rosenberg SA. Immunization of patients with the hTERT:540-548 peptide induces peptide-reactive T lymphocytes that do not recognize tumors endogenously expressing telomerase. Clin Cancer Res. 2004; 10:4688–4698. PMID: 15269141.
Article15. Raje N, Hideshima T, Davies FE, Chauhan D, Treon SP, Young G, et al. Tumour cell/dendritic cell fusions as a vaccination strategy for multiple myeloma. Br J Haematol. 2004; 125:343–352. PMID: 15086415.
Article16. Lee JJ, Choi BH, Kang HK, Park MS, Park JS, Kim SK, et al. Induction of multiple myeloma-specific cytotoxic T lymphocyte stimulation by dendritic cell pulsing with purified and optimized myeloma cell lysates. Leuk Lymphoma. 2007; 48:2022–2031. PMID: 17917970.
Article17. Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006; 29:545–557. PMID: 16971810.18. Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008; 68:5972–5978. PMID: 18632653.
Article19. Takei M, Tachikawa E, Hasegawa H, Lee JJ. Dendritic cells maturation promoted by M1 and M4, end products of steroidal ginseng saponins metabolized in digestive tracts, drive a potent Th1 polarization. Biochem Pharmacol. 2004; 68:441–452. PMID: 15242811.
Article20. Trepiakas R, Pedersen AE, Met O, Hansen MH, Berntsen A, Svane IM. Comparison of alpha-type-1 polarizing and standard dendritic cell cytokine cocktail for maturation of therapeutic monocyte-derived dendritic cell preparations from cancer patients. Vaccine. 2008; 26:2824–2832. PMID: 18450338.21. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000; 406:747–752. PMID: 10963602.
Article22. Porter DA, Krop IE, Nasser S, Sgroi D, Kaelin CM, Marks JR, et al. A SAGE (serial analysis of gene expression) view of breast tumor progression. Cancer Res. 2001; 61:5697–5702. PMID: 11479200.23. Matsumoto S, Saito H, Tsujitani S, Ikeguchi M. Allogeneic gastric cancer cell-dendritic cell hybrids induce tumor antigen (carcinoembryonic antigen) specific CD8+ T cells. Cancer Immunol Immunother. 2006; 55:131–139. PMID: 15891883.24. Delirezh N, Moazzeni SM, Shokri F, Shokrgozar MA, Atri M, Kokhaei P. Autologous dendritic cells loaded with apoptotic tumor cells induce T cell-mediated immune responses against breast cancer in vitro . Cell Immunol. 2009; 257:23–31. PMID: 19306994.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-tumor Cytotoxicity of Allogeneic Neuroblastoma Tumor Antigen-loaded Dendiritic Cells
- A Study for Apoptosis and Its Mechanism of Allogeneic Activated T Lymphocytes Induced by Mouse Liver Immature Dendritic Cells
- Glioma Stem Cell-Targeted Dendritic Cells as a Tumor Vaccine Against Malignant Glioma
- Current Approaches in Development of Immunotherapeutic Vaccines for Breast Cancer
- Invited Commentary: Role of Estrogen Receptor-alpha in Regulating Claudin-6 Expression in Breast Cancer Cells