Cancer Res Treat.
2004 Oct;36(5):324-329.
Phenylacetate Induces Growth Inhibition and Apoptosis of Human Osteosarcoma Cells
- Affiliations
-
- 1Department of Pathology, Chonbuk National University Medical School, Jeonju, Korea. mjkang@chonbuk.ac.kr
Abstract
- PURPOSE
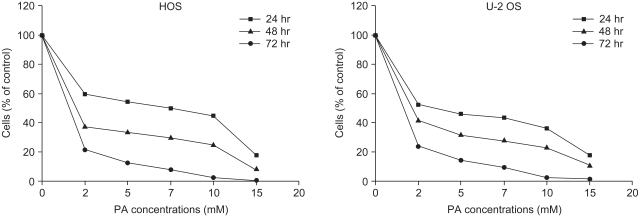
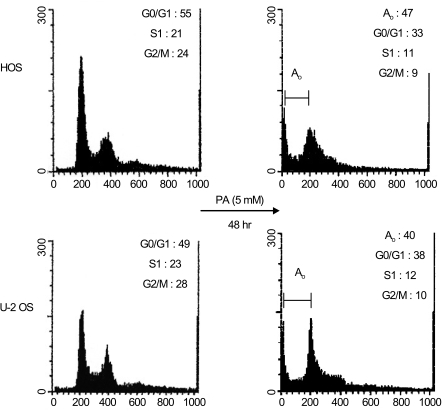
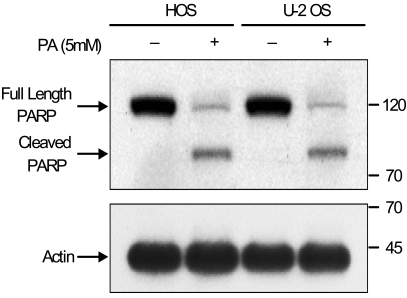
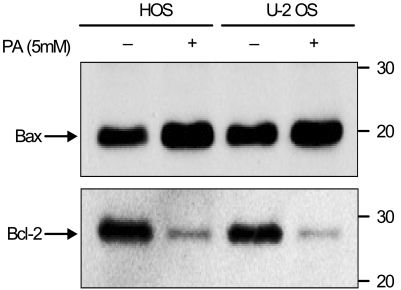
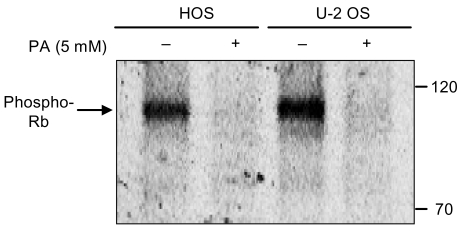
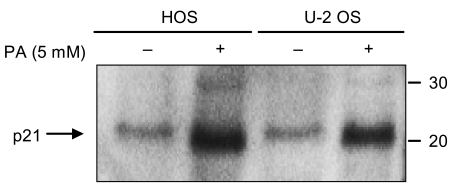
Phenylacetate has potent antiproliferative effects in many malignant tumors. However, the exact mechanism as to how phenylacetate induces cell growth arrest remains unclear and very little is known about its effects on human osteosarcoma cells. In this study, we investigated whether phenylacetate is effective against two osteosarcoma cell lines (HOS and U-2 OS) in vitro. MATERIALS AND METHODS: The viability of phenylacetate- treated cell lines was assessed by trypan blue exclusion assay, and the cell cycle distribution was measured by flow cytometry. To measure cell apoptosis, poly (ADP- ribose) polymerase cleavage assay and flow cytometry were employed. The expressions of cell cycle-regulatory proteins and the apoptosis-related genes were evaluated by western blot analysis. RESULTS: Phenylacetate was found to inhibit the growth of osteosarcoma cells, induce cell cycle arrest in the G1 phase, and induce apoptosis. A significant decrease in Bcl-2 expression and a mild up-regulation of Bax were also observed in both phenylacetate-treated cell lines. Reduced phosphorylation of the pRb and the increased expression of p21Cip1 were observed subsequent to treatment with phenylacetate. CONCLUSION: These findings support the idea that pheny lacetate may be an effective chemotherapeutic agent to be employed in the future against osteosarcoma, because phenylacetate acts to inhibit the growth of osteosarcoma cells through cell cycle arrest and apoptosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Wada T, Isu K, Takeda N, Usui M, Ishii S, Yamawaki S. A preliminary report of neoadjuvant chemotherapy NSH-7 study in osteosarcoma: preoperative salvage chemotherapy based on clinical tumor response and the use of granulocyte colony-stimulating factor. Oncology. 1996; 53:221–227. PMID: 8643225.
Article2. Sluga M, Windhager R, Lang S, Heinzl H, Bielack S, Kotz R. Local and systemic control after ablative and limb sparing surgery in patients with osteosarcoma. Clin Orthop. 1999; 358:120–127. PMID: 9973983.
Article3. Scotlandi K, Serra M, Nicoletti G, Vaccari M, Manara MC, Nini G, Landuzzi L, Colacci A, Bacci G, Bertoni F, Picci P, Campanacci M, Baldini N. Multidrug resistance and malignancy in human osteosarcoma. Cancer Res. 1996; 56:2434–2439. PMID: 8625324.4. Sandler M, Ruthven CR, Goodwin BL, Lees A, Stern GM. Phenylacetic acid in human body fluids: high correlation between plasma and cerebrospinal fluid concentration values. J Neurol Neurosurg Psychiatry. 1982; 45:366–368. PMID: 7077347.
Article5. Samid D, Shack S, Sherman LT. Phenylacetate: a novel nontoxic inducer of tumor cell differentiation. Cancer Res. 1992; 52:1988–1992. PMID: 1372534.6. Samid D, Shack S, Myers CE. Selective growth arrest and phenotypic reversion of prostate cancer cells in vitro by nontoxic pharmacological concentrations of phenylacetate. J Clin Invest. 1993; 91:2288–2295. PMID: 8486788.7. Adam L, Crepin M, Israel L. Tumor growth inhibition, apop tosis, and Bc1-2 down-regulation of MCF-7ras tumors by sodium phenylacetate and tamoxifen combination. Cancer Res. 1997; 57:1023–1029. PMID: 9067263.8. Harrison LE, Wojciechowicz DC, Brennan MF, Paty PB. Phenylacetate inhibits isoprenoid biosynthesis and suppresses growth of human pancreatic carcinoma. Surgery. 1998; 124:541–550. PMID: 9736908.
Article9. Call TG, Stenson MJ, Witzig TE. Effects of phenylacetate on cells from patients with B-chronic lymphocytic leukemia. Leuk Lymphoma. 1994; 14:145–149. PMID: 7920222.
Article10. Ferrandina G, Melichar B, Loercher A, Verschraegen CF, Kudelka AP, Edwards CL, Scambia G, Kavanagh JJ, Abbruzzese JL, Freedman RS. Growth inhibitory effects of sodium phenylacetate (NSC3039) on ovarian carcinoma cells in vitro. Cancer Res. 1997; 57:4309–4315. PMID: 9331092.11. Stockhammer G, Manley GT, Johnson R, Rosenblum MK, Samid D, Lieberman FS. Inhibition of proliferation and induction of differentiation in medulloblastoma- and astrocytoma-derived cell lines with phenylacetate. J Neurosurg. 1995; 83:672–681. PMID: 7674018.
Article12. Chiarugi V, Magnelli L, Cinelli M, Basi G. Apoptosis and the cell cycle. Cell Mol Biol Res. 1994; 40:603–612. PMID: 7787878.13. Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA. 1995; 92:7834–7838. PMID: 7644501.
Article14. Adam L, Crepin M, Savin C, Israel L. Sodium phenylacetate induces growth inhibition and Bcl-2 down-regulation and apoptosis in MCF7ras cells in vitro and in nude mice. Cancer Res. 1995; 55:5156–5160. PMID: 7585564.16. Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995; 81:323–330. PMID: 7736585.
Article17. Franco OE, Onishi T, Umeda Y, Soga N, Wakita T, Arima K, Yanagawa M, Sugimura Y. Phenylacetate inhibits growth and modulates cell cycle gene expression in renal cancer cell lines. Anticancer Res. 2003; 23:1637–1642. PMID: 12820434.18. Onishi T, Yamakawa K, Franco OE, Suzuki R, Kawamura J. p27Kip1 is the key mediator of phenylacetate induced cell cycle arrest in human prostate cancer cells. Anticancer Res. 2000; 20:3075–3081. PMID: 11062725.19. Medina MA, Sanchez-Jimenez F, Marquez FJ, Perez-Rodriguez J, Quesada AR, Nunez de Castro I. Glutamine and glucose as energy substrates for Ehrlich ascites tumour cells. Biochem Int. 1988; 16:339–347. PMID: 3365266.20. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990; 343:425–430. PMID: 1967820.
Article22. Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994; 79:1147–1156. PMID: 8001151.23. La Thangue NB. DRTF1/E2F: an expanding family of heterodimeric transcription factors implicated in cell-cycle control. Trends Biochem Sci. 1994; 19:108–114. PMID: 8203017.
Article24. Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995; 15:2612–2624. PMID: 7739542.
Article25. Gorospe M, Shack S, Guyton KZ, Samid D, Holbrook NJ. Up-regulation and functional role of p21Waf1/Cip1 during growth arrest of human breast carcinoma MCF-7 cells by phenylacetate. Cell Growth Differ. 1996; 7:1609–1615. PMID: 8959328.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Over-expression of PTEN Involved in Troglitazone-induced Apoptosis in Human Osteosarcoma Cells
- Chios Gum Mastic Induces Cell Cycle Arrest and Apoptosis in Human Osteosarcoma Cells
- Autophagy Inhibition Promotes Quercetin Induced Apoptosis in MG-63 Human Osteosarcoma cells
- Knockdown of Long Non-Coding RNA NEAT1 Inhibits Proliferation and Invasion and Induces Apoptosis of Osteosarcoma by Inhibiting miR-194 Expression
- The Effect of Disodium Pamidronate on Human Osteosarcoma Cells