Cancer Res Treat.
2004 Oct;36(5):315-323.
Activity of Green Tea Polyphenol Epigallocatechin-3-gallate Against Ovarian Carcinoma Cell Lines
- Affiliations
-
- 1Department of Obstetrics and Gynecology, The Catholic University of Korea, Seoul, Korea. ahnws@catholic.ac.kr
- 2Catholic Research Institutes of Medical Science College of Medicine, The Catholic University of Korea, Seoul, Korea.
- 3Department Obstetrics and Gynecology, College of Medicine, Chosun University, Gwang-ju, Korea.
- 4Department of Food Function Research, Korea Food Research Institute, Korea.
- 5Department of Bioscience and Biotechnology, SeJong University, Seoul, Korea.
- 6College of Pharmacy, Seoul National University, Seoul, Korea.
Abstract
- PURPOSE
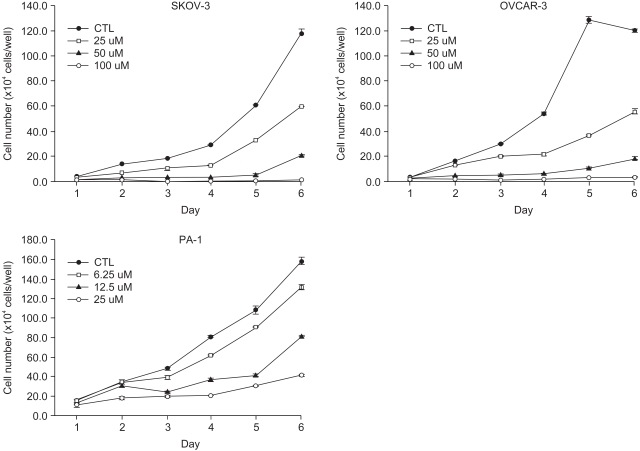
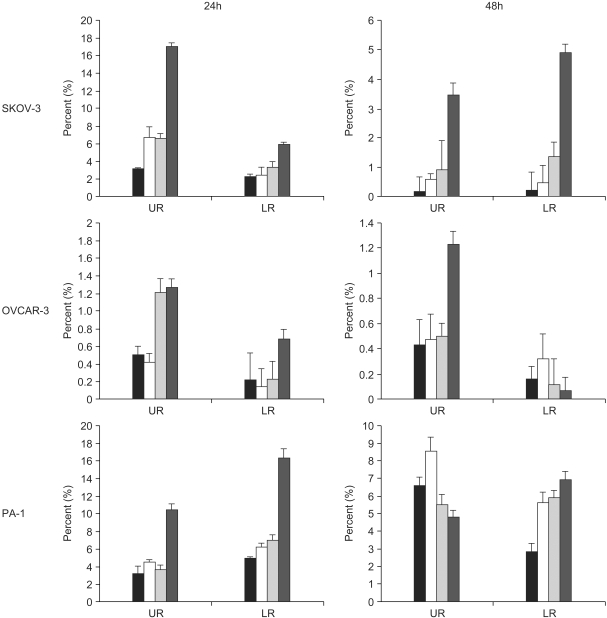
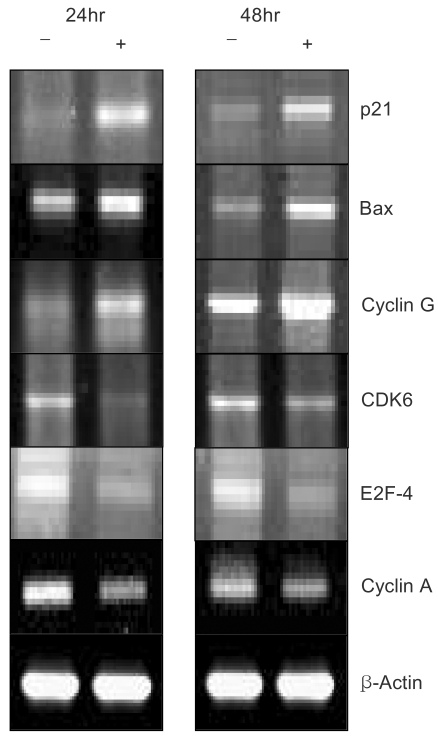
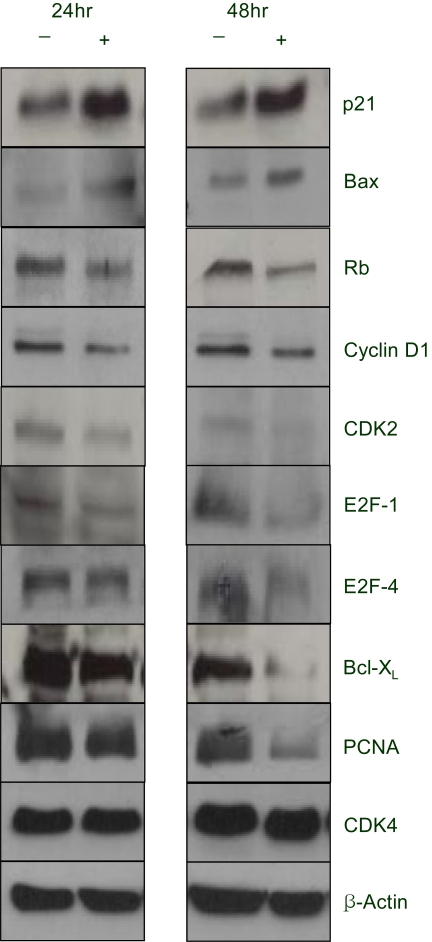
A constituent of green tea, (-)-epigallocatechin-3-gallate (EGCG), is known to possess anti-cancer properties. In this study, the time-course of the anticancer effects of EGCG on human ovarian cancer cells were investigated to provide insights into the molecular-level understanding of the growth suppression mechanism involved in EGCG-mediated apoptosis and cell cycle arrest. MATERIALS AND METHODS: Three human ovarian cancer cell lines (p53 negative, SKOV-3 cells; mutant type p53, OVCAR-3 cells; and wild type p53, PA-1 cells) were used. The effect of EGCG treatment was studied via a cell count assay, cell cycle analysis, FACS, Western blot and macroarray assay. RESULTS: EGCG exerts a significant role in suppressing ovarian cancer cell growth, showed dose dependent growth inhibitory effects in each cell line and induced apoptosis and cell cycle arrest. The cell cycle was arrested at the G1 phase by EGCG in SKOV-3 and OVCAR-3 cells. In contrast, the cell cycle was arrested in the G1/S phase in PA-1 cells. EGCG differentially regulated the expression of genes and proteins (Bax, p21, Retinoblastoma, cyclin D1, CDK4 and Bcl-XL) more than 2 fold, showing a possible gene regulatory role for EGCG. The continual expression in p21WAF1 suggests that EGCG acts in the same way with p53 proteins to facilitate apoptosis after EGCG treatment. Bax, PCNA and Bcl-X are also important in EGCG-mediated apoptosis. In contrast, CDK4 and Rb are not important in ovarian cancer cell growth inhibition. CONCLUSION: EGCG can inhibit ovarian cancer cell growth through the induction of apoptosis and cell cycle arrest, as well as in the regulation of cell cycle related proteins. Therefore, EGCG-mediated apoptosis could be applied to an advanced strategy in the development of a potential drug against ovarian cancer.
MeSH Terms
Figure
Reference
-
1. Newman B, Millikan RC, King MC. Genetic epidemiology of breast and ovarian cancers. Epidemiol Rev. 1997; 19:69–79. PMID: 9360904.
Article2. Kim JS, Bae JS, Kim KH, Ahn CH, Oh SJ, Jeon HM, Lim KW, Chun CS. Clinical Analysis of PTEN, p53 and Her-2/neu Expressions in Thyroid Cancers. Cancer Res Treat. 2001; 33:433–437.
Article3. Kitano H. Tumour tactics. Nature. 2003; 426:125. PMID: 14614483.4. Ji BT, Chow WH, Hsing AW, McLaughlin JK, Dai Q, Gao YT, Blot WJ, Fraumeni JF Jr. Green tea consumption and the risk of pancreatic and colorectal cancers. Int J Cancer. 1997; 70:255–258. PMID: 9033623.
Article5. Kato I, Tominaga S, Matsuura A, Yoshii Y, Shirai M, Kobayashi S. A comparative case-control study of colorectal cancer and adenoma. Jpn J Cancer Res. 1990; 81:1101–1108. PMID: 2125036.
Article6. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997; 26:769–775. PMID: 9388788.
Article7. Asano Y, Okamura S, Ogo T, Eto T, Otsuka T, Niho Y. Effects of (-)-epigallocatechin gallate on leukemic blast cells from patients with acute myeloblastic leukemia. Life Sci. 1997; 60:135–142. PMID: 9000119.8. Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epogallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997; 89:1881–1886. PMID: 9414176.9. Gao YT, McLaughlin JK, Blot WJ, Ji BT, Dai Q, Fraumeni JF Jr. Reduced risk of esophageal cancer associated with green tea consumption. J Natl Cancer Inst. 1994; 86:855–858. PMID: 8182766.
Article10. Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997; 387:561. PMID: 9177339.
Article11. Yang CS, Wang ZY. Tea and cancer. J Natl Cancer Inst. 1993; 85:1038–1049. PMID: 8515490.
Article12. Komori A, Yatsunami J, Okabe S, Abe S, Hara K, Sugamura M, Kim SJ, Fujiki H. Anti-carcinogenic activity of green tea polyphenols. J Cancer Res Clin Oncol. 1993; 23:186–190.13. Paschka AG, Butler R, Young CYF. Induction of apoptosis in prostate cancer cell lines by the green tea component, (-)-epigallocatechin-3 gallate. Cancer Letters. 1998; 130:1–7. PMID: 9751250.14. Anderson RF, Fisher LJ, Hara Y, Harris T, Mak WB, Melton LD, Packer JE. Green tea catechins partially protect DNA from OH radical-induced strand breaks and base damage through fast chemical repair of DNA radicals. Carcinogenesis. 2001; 22:1189–1193. PMID: 11470748.15. Anderson RF, Amarasinghe C, Fisher LJ, Mak WB, Packer JE. Reduction in free-radical-induced DNA strand breaks and base damage through fast chemical repair by flavonoids. Free Radic Res. 2000; 33:91–103. PMID: 10826925.
Article16. Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000; 71:1698S–1702S. PMID: 10837321.
Article17. Kavanagh KT, Hafer LJ, Kim DW, Mann KK, Sherr DH, Rogers AE, Sonenshein GE. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J Cell Biochem. 2001; 82:387–398. PMID: 11500915.
Article18. Jung YD, Ellis LM. Inhibition of tumour invasion and angiogenesis by epigallocatechin gallate (EGCG), a major component of green tea. Int J Exp Pathol. 2001; 82:309–316. PMID: 11846837.
Article19. Ahmad N, Cheng P, Mukhtar H. Cell cycle dysregulation by green tea polyphenol epigallocatechin-3-gallate. Biochem Biophys Res Commun. 2000; 275:328–334. PMID: 10964666.
Article20. Otsuka T, Ogo T, Eto T, Asano Y, Suganuma M, Niho Y. Growth inhibition of leukemic cells by(-) epogallocatechin gallate, the main constituent of green tea. Life Sci. 1998; 63:1397–1403. PMID: 9952285.21. Yang GY, Liao J, Kim KH, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998; 19:611–616. PMID: 9600345.
Article22. Suganuma M, Okabe S, Sucoka N, Sueoka E, Matsuyama E, Imai K, Nakachi K, Fujiki H. Green tea and cancer chemoprevention. Mutation Res. 1999; 428:339–344. PMID: 10518005.
Article23. Okabe S, Ochiai Y, Aida M, Park K, Kim SJ, Nomura T, Suganuma M, Fujiki H. Mechanistic aspects of green tea as a cancer preventive: effect of components on human stomach cancer cell lines. Jpn J Cancer Res. 1999; 90:733–739. PMID: 10470285.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts
- Effects of Green tea (-)-epigallocatechin-3-gallate and Polyphenol on the Proliferation and Apoptosis of Cultured Human Keratinocytes, Melanocytes, Fibroblasts, Endothelial Cells, and Human Epidermoid Carcinoma Cells
- A Case of Green Tea-induced Occupational Asthma
- Effect of Extraction Conditions of Green Tea on Antioxidant Activity and EGCG Content: Optimization using Response Surface Methodology
- The Effect of Delayed Administration of Green Tea Polyphenol, (-)-pigallocatechin-3-gallate, on the Change of Putrescine Level and Hippocampal Neuronal Cell Damage after Transient Global Ischemia in Gerbil