Ann Surg Treat Res.
2015 Feb;88(2):55-62. 10.4174/astr.2015.88.2.55.
Effects of combination therapy of docetaxel with selenium on the human breast cancer cell lines MDA-MB-231 and MCF-7
- Affiliations
-
- 1Department of Emergency Medicine, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 2Department of Surgery, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea. kspark@kuh.ac.kr
- 3Department of Surgery, Konkuk University Chungju Hospital, Chungju, Korea.
- 4Department of Emergency Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Emergency Medicine, Dongguk University Ilsan Hospital, Dongguk University College of Medicine, Goyang, Korea.
- KMID: 1804121
- DOI: http://doi.org/10.4174/astr.2015.88.2.55
Abstract
- PURPOSE
The anticancer property and cytoprotective role of selenium in chemotherapy have been reported. However, the combination effects of selenium on chemotherapy for advanced breast cancer have not yet been clearly defined. The purpose of this study was to investigate the combined effects of selenium on chemotherapy using docetaxel on breast cancer cell lines.
METHODS
Under adherent culture conditions, two breast cancer cell lines, MDA-MB-231 and MCF-7, were treated with docetaxel at 500pM and selenium at 100nM, 1microM, or 10microM. Changes in cell growth, cell cycle duration, and degree of apoptosis after 72 hours in each treated group were evaluated.
RESULTS
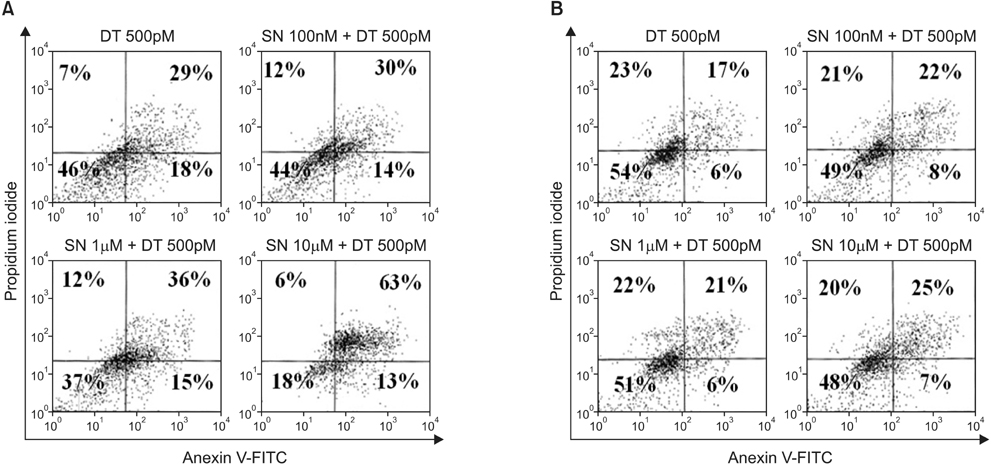
In the MDA-MB-231 cells, the combination therapy group (docetaxel at 500pM plus selenium at 10microM) showed a significantly decreased percentage of cell growth (15% vs. 28%; P = 0.004), a significantly increased percentage of late apoptosis (63% vs. 26%; P = 0.001), and an increased cell cycle arrest in the G2/M phase (P = 0.001) compared with the solitary docetaxel therapy group. Isobologram analysis demonstrated the synergistic effect of the combination therapy in the MDA-MB-231 cells. However, in the MCF-7 cells, no significant differences in the percentage of cell growth apoptosis, the percentage of apoptosis, and the pattern of cell cycle arrest were noted between the combination therapy groups and the solitary docetaxel therapy group.
CONCLUSION
Our in vitro study indicated that the combination of selenium with docetaxel inhibits cell proliferation through apoptosis and cell arrest in the G2/M phase in MDA-MB-231 breast cancer cells.
MeSH Terms
Figure
Reference
-
1. Ko BS, Noh WC, Kang SS, Park BW, Kang EY, Paik NS, et al. Changing patterns in the clinical characteristics of korean breast cancer from 1996-2010 using an online nationwide breast cancer database. J Breast Cancer. 2012; 15:393–400.2. Leclere B, Molinie F, Tretarre B, Stracci F, Daubisse-Marliac L, Colonna M, et al. Trends in incidence of breast cancer among women under 40 in seven European countries: a GRELL cooperative study. Cancer Epidemiol. 2013; 37:544–549.3. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.4. Yvon AM, Wadsworth P, Jordan MA. Taxol suppresses dynamics of individual microtubules in living human tumor cells. Mol Biol Cell. 1999; 10:947–959.5. Kleeberg UR, Fink M, Tessen HW, Nennecke A, Hentschel S, Bartels S. Adjuvant therapy reduces the benefit of palliative treatment in disseminated breast cancer: own findings and review of the literature. Onkologie. 2013; 36:348–356.6. Valero V, Holmes FA, Walters RS, Theriault RL, Esparza L, Fraschini G, et al. Phase II trial of docetaxel: a new, highly effective antineoplastic agent in the management of patients with anthracycline-resistant metastatic breast cancer. J Clin Oncol. 1995; 13:2886–2894.7. Martin M, Segui MA, Anton A, Ruiz A, Ramos M, Adrover E, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2010; 363:2200–2210.8. Baker J, Ajani J, Scotte F, Winther D, Martin M, Aapro MS, et al. Docetaxel-related side effects and their management. Eur J Oncol Nurs. 2009; 13:49–59.9. Hamilton SJ. Review of selenium toxicity in the aquatic food chain. Sci Total Environ. 2004; 326:1–31.10. Harris HR, Bergkvist L, Wolk A. Selenium intake and breast cancer mortality in a cohort of Swedish women. Breast Cancer Res Treat. 2012; 134:1269–1277.11. Holmes MD, Stampfer MJ, Colditz GA, Rosner B, Hunter DJ, Willett WC. Dietary factors and the survival of women with breast carcinoma. Cancer. 1999; 86:826–835.12. Watrach AM, Milner JA, Watrach MA, Poirier KA. Inhibition of human breast cancer cells by selenium. Cancer Lett. 1984; 25:41–47.13. Erickson VS, Pearson ML, Ganz PA, Adams J, Kahn KL. Arm edema in breast cancer patients. J Natl Cancer Inst. 2001; 93:96–111.14. Letavayova L, Vlckova V, Brozmanova J. Selenium: from cancer prevention to DNA damage. Toxicology. 2006; 227:1–14.15. Boucher F, Coudray C, Tirard V, Barandier C, Tresallet N, Favier A, et al. Oral selenium supplementation in rats reduces cardiac toxicity of adriamycin during ischemia and reperfusion. Nutrition. 1995; 11:5 Suppl. 708–711.16. Fujieda M, Naruse K, Hamauzu T, Miyazaki E, Hayashi Y, Enomoto R, et al. Effect of selenium on Cisplatin-induced nephrotoxicity in rats. Nephron Exp Nephrol. 2006; 104:e112–e122.17. Francescato HD, Costa RS, Rodrigues Camargo SM, Zanetti MA, Lavrador MA, Bianchi MD. Effect of oral selenium administration on cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2001; 43:77–82.18. Ohkawa K, Tsukada Y, Dohzono H, Koike K, Terashima Y. The effects of co-administration of selenium and cisplatin (CDDP) on CDDP-induced toxicity and antitumour activity. Br J Cancer. 1988; 58:38–41.19. Tan L, Jia X, Jiang X, Zhang Y, Tang H, Yao S, et al. In vitro study on the individual and synergistic cytotoxicity of adriamycin and selenium nanoparticles against Bel7402 cells with a quartz crystal microbalance. Biosens Bioelectron. 2009; 24:2268–2272.20. Baliga MS, Wang H, Zhuo P, Schwartz JL, Diamond AM. Selenium and GPx-1 overexpression protect mammalian cells against UV-induced DNA damage. Biol Trace Elem Res. 2007; 115:227–242.21. Kellen E, Zeegers M, Buntinx F. Selenium is inversely associated with bladder cancer risk: a report from the Belgian case-control study on bladder cancer. Int J Urol. 2006; 13:1180–1184.22. van den Brandt PA, Goldbohm RA, van't Veer P, Bode P, Dorant E, Hermus RJ, et al. A prospective cohort study on selenium status and the risk of lung cancer. Cancer Res. 1993; 53:4860–4865.23. Peters U, Chatterjee N, Church TR, Mayo C, Sturup S, Foster CB, et al. High serum selenium and reduced risk of advanced colorectal adenoma in a colorectal cancer early detection program. Cancer Epidemiol Biomarkers Prev. 2006; 15:315–320.24. Weijl NI, Cleton FJ, Osanto S. Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat Rev. 1997; 23:209–240.25. Zimmermann T, Leonhardt H, Kersting S, Albrecht S, Range U, Eckelt U. Reduction of postoperative lymphedema after oral tumor surgery with sodium selenite. Biol Trace Elem Res. 2005; 106:193–203.26. Suzuki M, Endo M, Shinohara F, Echigo S, Rikiishi H. Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother Pharmacol. 2010; 66:475–484.27. Luo H, Wang F, Bai Y, Chen T, Zheng W. Selenium nanoparticles inhibit the growth of HeLa and MDA-MB-231 cells through induction of S phase arrest. Colloids Surf B Biointerfaces. 2012; 94:304–308.28. Rao L, Puschner B, Prolla TA. Gene expression profiling of low selenium status in the mouse intestine: transcriptional activation of genes linked to DNA damage, cell cycle control and oxidative stress. J Nutr. 2001; 131:3175–3181.29. Zhang S, Li F, Younes M, Liu H, Chen C, Yao Q. Reduced selenium-binding protein 1 in breast cancer correlates with poor survival and resistance to the anti-proliferative effects of selenium. PLoS One. 2013; 8:e63702.30. Vekariya KK, Kaur J, Tikoo K. ERa signaling imparts chemotherapeutic selectivity to selenium nanoparticles in breast cancer. Nanomedicine. 2012; 8:1125–1132.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Docetaxel-loaded PLGA nanoparticles to increase pharmacological sensitivity in MDA-MB-231 and MCF-7 breast cancer cells
- Ellagic Acid Shows Different Anti-proliferative Effects Between the MDA-MB-231 and MCF-7 Human Breast Cancer Cell Lines
- Comparative Studies on the Polyarnine Involvement in MCF - 7 and MDA - MB - 231 Breast Cancer Cell Proliferation
- Potentiation of the Anticancer Effects by Combining Docetaxel with Ku-0063794 against Triple-Negative Breast Cancer Cells
- Dioscin Decreases Breast Cancer Stem-like Cell Proliferation via Cell Cycle Arrest by Modulating p38 Mitogen-activated Protein Kinase and AKT/mTOR Signaling Pathways